One of the fundamental questions for someone interested in the impact of doubled CO2 is exactly how (1) the greenhouse effect works; and (2) how the “enhanced” greenhouse effect works. AR4 FAQ 3.1 poses the question:

I’m going to show their answer to this question in full because the answer does not rise about a primary school level and can hardly be considered an adequate answer to the question. (And it’s not answered in AR1, AR2 or AR3 either.) While I think that this is the sort of thing that should be laid in detail in one of the reports, I could understand if they chose to refer interested readers to texts containing expositions that met IPCC standards. But no luck there. We simply get a grade school brochure without references.
AR4 FAQ 3.1
Here’s how IPCC “explained” the greenhose effect.
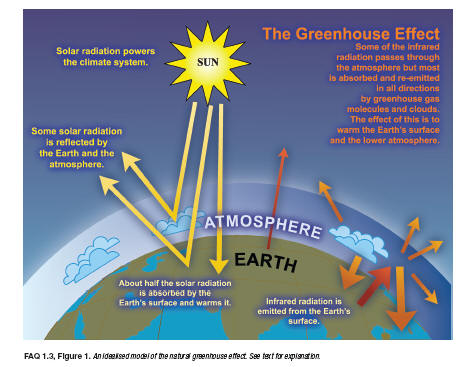
To balance the absorbed incoming energy, the Earth must, on average, radiate the same amount of energy back to space. Because the Earth is much colder than the Sun, it radiates at much longer wavelengths, primarily in the infrared part of the spectrum (see Figure 1). Much of this thermal radiation emitted by the land and ocean is absorbed by the atmosphere, including clouds, and reradiated back to Earth. This is called the greenhouse effect. The glass walls in a greenhouse reduce airflow and increase the temperature of the air inside. Analogously, but through a different physical process, the Earth’s greenhouse effect warms the surface of the planet. Without the natural greenhouse effect, the average temperature at Earth’s surface would be below the freezing point of water. Thus, Earth’s natural greenhouse effect makes life as we know it possible. However, human activities, primarily the burning of fossil fuels and clearing of forests, have greatly intensified the natural greenhouse effect, causing global warming.
The two most abundant gases in the atmosphere, nitrogen (comprising 78% of the dry atmosphere) and oxygen (comprising 21%), exert almost no greenhouse effect. Instead, the greenhouse effect comes from molecules that are more complex and much less common. Water vapour is the most important greenhouse gas, and carbon dioxide (CO2) is the second-most important one. Methane, nitrous oxide, ozone and several other gases present in the atmosphere in small amounts also contribute to the greenhouse effect. In the humid equatorial regions, where there is so much water vapour in the air that the greenhouse effect is very large, adding a small additional amount of CO2 or water vapour has only a small direct impact on downward infrared radiation. However, in the cold, dry polar regions, the effect of a small increase in CO2 or water vapour is much greater. The same is true for the cold, dry upper atmosphere where a small increase in water vapour has a greater influence on the greenhouse effect than the same change in water vapour would have near the surface.
Adding more of a greenhouse gas, such as CO2, to the atmosphere intensifies the greenhouse effect, thus warming Earth’s climate. The amount of warming depends on various feedback mechanisms. For example, as the atmosphere warms due to rising levels of greenhouse gases, its concentration of water vapour increases, further intensifying the greenhouse effect. This in turn causes more warming, which causes an additional increase in water vapour, in a self-reinforcing cycle. This water vapour feedback may be strong enough to approximately double the increase in the greenhouse effect due to the added CO2 alone.
Additional important feedback mechanisms involve clouds. Clouds are effective at absorbing infrared radiation and therefore exert a large greenhouse effect, thus warming the Earth. Clouds are also effective at reflecting away incoming solar radiation, thus cooling the Earth. A change in almost any aspect of clouds, such as their type, location, water content, cloud altitude, particle size and shape, or lifetimes, affects the degree to which clouds warm or cool the Earth. Some changes amplify warming while others diminish it. Much research is in progress to better understand how clouds change in response to climate warming, and how these changes affect climate through various feedback mechanisms.
I’m not suggesting that this brochure is wrong, but surely stadiums full of the best climate science minds could have produced something a little more sophisticated than this. And it’s not that the topics are handled in an unsophisticated way in (say) Goody and Yung. The problem is that IPCC chose not to provide an adequate exposition of a fundamental topic under discussion.
So when realclimate scientists get annoyed at the public’s seeming inability to understand some issues, they should probably spend a little less time blaming others and a little more time looking in the mirror and evaluating whether they properly discharged their obligations when this sort of pablum was published in their most influential presentation. How is a scientist from another discipline supposed to come away from this brochure with an understanding of the mechanism or where he can find an approved text exposition, if that’s the alternative.
IPCC TAR
Ah, you might say that this was already explained in an earlier report. Well, IPCC TAR contained a similar pablum-style exposition, shown below – also without references even to a text book. The TAR paragraph also contains a mention of “the higher the colder” heuristic, also used in Houghton’s text (Which I’ll discuss soon).
The natural greenhouse effect
The atmosphere contains several trace gases which absorb and emit infrared radiation. These so-called greenhouse gases absorb infrared radiation, emitted by the Earth’s surface, the atmosphere and clouds, except in a transparent part of the spectrum called the “atmospheric window’, as shown in Figure 1.2. They emit in turn infrared radiation in all directions including downward to the Earth’s surface. Thus greenhouse gases trap heat within the atmosphere. This mechanism is called the natural greenhouse effect. The net result is an upward transfer of infrared radiation from warmer levels near the Earth’s surface to colder levels at higher altitudes. The infrared radiation is effectively radiated back into space from an altitude with a temperature of, on average, -19’C, in balance with the incoming radiation, whereas the Earth’s surface is kept at a much higher temperature of on average 14’C. This effective emission temperature of -19’C corresponds in mid-latitudes with a height of approximately 5 km. Note that it is essential for the greenhouse effect that the temperature of the lower atmosphere is not constant (isothermal) but decreases with height.The natural greenhouse effect is part of the energy balance of the Earth, as can be seen schematically in Figure 1.2.
Figure 1.2: The Earth’s annual and global mean energy balance. Of the incoming solar radiation, 49% (168 Wm-2) is absorbed by the surface. That heat is returned to the atmosphere as sensible heat, as evapotranspiration (latent heat) and as thermal infrared radiation. Most of this radiation is absorbed by the atmosphere, which in turn emits radiation both up and down. The radiation lost to space comes from cloud tops and atmospheric regions much colder than the surface. This causes a greenhouse effect. Source: Kiehl and Trenberth, 1997: Earth’s Annual Global Mean Energy Budget, Bull. Am. Met. Soc. 78, 197-208.
TAR included the following “exposition” of the enhanced greenhouse effect also without any references even to a textbook. While it states the “enhanced greenhouse effect” is discussed “in detail” in Chapter 6, this proves not to be the case.
(1.3.1) The enhanced greenhouse effect
The increased concentration of greenhouse gases in the atmosphere enhances the absorption and emission of infrared radiation. The atmosphere’s opacity increases so that the altitude from which the Earth’s radiation is effectively emitted into space becomes higher. Because the temperature is lower at higher altitudes, less energy is emitted, causing a positive radiative forcing. This effect is called the enhanced greenhouse effect, which is discussed in detail in Chapter 6.If the amount of carbon dioxide were doubled instantaneously, with everything else remaining the same, the outgoing infrared radiation would be reduced by about 4 Wm-2. In other words, the radiative forcing corresponding to a doubling of the CO2 concentration would be 4 Wm-2. To counteract this imbalance, the temperature of the surface-troposphere system would have to increase by 1.2’C (with an accuracy of “10%), in the absence of other changes. In reality, due to feedbacks, the response of the climate system is much more complex. It is believed that the overall effect of the feedbacks amplifies the temperature increase to 1.5 to 4.5’C. A significant part of this uncertainty range arises from our limited knowledge of clouds and their interactions with radiation. To appreciate the magnitude of this temperature increase, it should be compared with the global mean temperature difference of perhaps 5 or 6’C from the middle of the last Ice Age to the present interglacial.
The so-called water vapour feedback, caused by an increase in atmospheric water vapour due to a temperature increase, is the most important feedback responsible for the amplification of the temperature increase. Concern has been expressed about the strength of this feedback, in particular in relation to the role of upper tropospheric humidity. Since the SAR, thinking about this feedback has become increasingly sophisticated thanks both to modelling and to observational studies. Feedbacks are discussed and assessed in Chapter 7. In particular, the present state of knowledge of the water vapour feedback is examined in Section 7.2.1.
It has been suggested that the absorption by CO2 is already saturated so that an increase would have no effect. This, however, is not the case. Carbon dioxide absorbs infrared radiation in the middle of its 15 mm band to the extent that radiation in the middle of this band cannot escape unimpeded: this absorption is saturated. This, however, is not the case for the band’s wings. It is because of these effects of partial saturation that the radiative forcing is not proportional to the increase in the carbon dioxide concentration but shows a logarithmic dependence. Every further doubling adds an additional 4 Wm-2 to the radiative forcing.
I’m not saying that an explanation is impossible, only documenting that IPCC TAR and AR4 failed to provide one. The general public should not be required to wade through Goody and Yung at a university library to get an explanation.





154 Comments
Steve:
A couple of related questions: Is the IPCC supposed to interface with, and supply information to the public, or with and to their UN superiors? I fully agree with your premise that many of these particulars of the climate change discussion could and should be explained in a more exact and thorough way, but is that part of their mandate? It is easy to get wrapped around the axle here and overlook the purpose of this UN panel, and expect more of them than they are mandated to provide. Are these FAQs part of their ‘job’ or just there to try to fend off all the constant stream of inquiries? IMHO, the UN track record is not exactly sterling in thinking things all the way through.
From header IPCC quote at the end –
This is not the spectroscopy that I remember being taught. I might have forgotten, or knowledge might have advanced.
Scenario: Photons of various energies are travelling upwards in the IR range. A photon can meet a molecule or atom, interact with it (or not), and raise the particle to a higher energy state. This higher state will usually be unstable and will revert to a ground state (for the surrounding conditions) typically by emission of a less energetic photon, plus heat.
Atmospheric CO2 does not act like a selective capture machine, waiting for photons at the middle of an absorption band and rejecting others. An absorption band, being a main one or a wing, exists because of its probability of interaction. If an incoming photon is able to interact probabilistically at the frequency of a wing, it will do so. The wings are a less probable target so they will be involved less often, but I cannot comprehend what this has to do with causation of a logarithmic relationship. They can become saturated just as much as any absorption frequency, I believe.
They issue press releases and have news conferences, so they are definitely disseminating information to the public.
I think that explaining the greenhouse effect and the enhanced greenhouse effect are certainly not inconsistent with their mandate. But regardless of their mandate, they elected to venture into this particular FAQ (in both reports).
As so often they veered from a highly professional literature review to a promotional and juvenile FAQ brochure. Had they done a better job on this, the level of public understanding would be higher. I find some of the comments on the greenhouse effect posted up by readers here to be quite annoying. While the topic may be crystal clear to climate scientists, this sort of explanation is much too elementary and accomplishes nothing.
Nothing, actually. The logarithmic relationship is no different than an attenuation placed in line with a speaker. There’s an upper limit to the amount of power that can be captured so the relationship cannot be linear (literally, I mean a straight line), and must have a monotonically decreasing slope. It’s pretty straightforward to figure out why it works this way, though the specifics are in dire need of explanation.
Mark
Steve: you’ve provided an analogy. The logarithmic relationship is plausible but that it holds for a speaker hardly makes it so for climate. See the new thread on logarithms please.
0.6 out of 33 is “greatly intensified”?
If the CO2 did not have wings it could not fly.
Steve M,
I strongly support your efforts overall, but seriously, what is your gripe here? This is the standard qualitative explanation for public consumption, and it’s largely uncontroversial.
The devil is in the details, of course.
The question is not one of basic physics, as RC would have one believe, but how myriad physical processes interact with one another.
You are not going to get an engineering explanation because the best AGW answer is a bit of basic physics, plus enough arm-waving to electrify to a small town. You are asking for blood from a stone – perhaps that is your point.
Maybe “intensely greatified” is the more correct phrase. 😉
Oh dear.
Fig.1.2 does not show up on my screen, but here it is:
One question. It is indicated that Earth surface adsorbs 168 W/m^2 of solar radiation in visible light region, but emits 390 W/m^2 in LW region (from which 324 W/m^2 is returned as back radiation).
Was this confirmed by direct measurements? Even at simplest case of tropical ocean on cloudless night?
The basics of the GHE and EGHE are the nub of the issue for me, so I’m happy to see a thread devoted to them. I used to believe these were well established theories, uncontroversial stuff, and my main skepticism centered around a climate system with high sensitivity. However I’ve read alot in the last six months that suggests the basic mechanisms were far from certain. I too came across these IPCC explanations, and find them pretty poor.
My main concerns, which I’d like to see possible answers for:
1. GHE seem to be dominated by radiative transfers, in reality I would expect the atmosphere to be dominated by convection and latent heat transfers, were is the proof that the GHE is reasonable?
2. EGHE, where atmospheric opacity to LWR raises height of topopause, reduces temp of photosphere, causes temperature rise at surface: this seems like a case of the tail wagging the dog; dare I use the term unphysical. Again where is the proof or evidence that this effect is real?
3. 33’C effect of GHE: seems like a strange argument. The real comparison would not be to an airless planet, but one with an atmosphere of just oxygen and nitrogen. The impression I get is that we should infer such a system, because the atmosphere would be transparent to LWR, would behave like a planet without an atmosphere; whereas surely it would behave as I mentioned in my first question: with convection dominating; I would expect such an atmosphere would still moderate the surface temperatures in the day by cooling through convection, and insulate at night; and of course this atmosphere would still be capable of radiating away it’s excess energy. To me this is a fundamental comparison that should be modeled, and I find it bizarre that it’s never mentioned and instead we are pointed at a totally meaningless comparison instead, why?
In summary, there seems no attempt at proving GHE by making predictions, and indicating how it might be falsified. Instead it is presented as a fait accompli, and it makes me nervous. Also it is the one part of Steve’s 2.5’C exposition that we should be able to get a handle on.
Gary Moran #11
Forget the 33°C . They represent nothing , are irrelevant and unphysical . They don’t even represent a zeroth approximation model . That has been shown already many times .
However your second remark is an interesting one . I have also been looking everywhere for a simulation of a planet with non IR active atmosphere (f.ex nitrogen only) . Unfortunately that doesn’t seem to have been done .
Indeed such a planet would probably be very violent .
The night half would cool extremely fast by radiating everything towards 0 K while the day half would heat fast too .
The daily min/max differentials would be very large .
The horizontal temperature gradients would be bigger than what we have while it is not so clear what the vertical gradients would be .
In detail the large scale circulation structure would depend on the rotation speed of the planet so it is not easy to imagine the result without some more serious modelling .
In any case such a model would be interesting to understand what the difference between an IR active and IR inactive atmosphere would be .
I think mr McIntyre has a good point going on and on why there isn’t a proper explanation even in the non-SPM versions of the IPCC reports. At first I though, he was just bashing IPCC a bit, but no one would expect them to be in the IPCC report.
The thing is that the GCM use this. That is, they model some sort of behaviour. But what behaviour? And do they all model the same behaviour? We know that the models differ strongly on things like aerosole cooling and sensitivity, but do they differ on the (other) fundamentals?
Also, when ‘sceptics’ object to the dominant behaviour of CGM in teh AGW theory, they always get thrown back that the theory rests on much more. However other than the measurements and reconstructions, then where is it? Where is this theory?
Or more general – and I don’t mean this joking – is there actually consensus on the theory?
#11, Gary Moran:
Actually, a discussion on GHE & EGHE is also going on at http://www.climateaudit.org/?p=2562 .
To respond to your specific questions:
1) The calculation of the GHE depends on both radiative transfer and convection. In brief, the temperature profile of the atmosphere is set by convection & latent-heat considerations (=> adiabatic lapse rate); based upon that temperature profile, the radiative transfer processes give rise to the radiative forcing which is the GHE.
2) As you say, when more GHG is added, the altitude of the photosphere rises (I don’t see that the tropopause would rise), and its temperature is reduced: so less power must be radiated away. Since just as much solar power is coming in, there must now be an imbalance, resulting in a warming up of the system. I think the most intuitive way to see that is to divide the IR radiation into upward/outward going and downward/inward going. Instantaneously after adding the extra GHG, the temperature profile of the atmosphere has not yet changed, so the upward radiation, which is sourced by the ground and the lower atmosphere, remains the same within the bounds of the old photosphere. But the downward radiation, which is sourced by the upper atmosphere, does not: At the radius of the old photosphere, before the addition of the GHG, the downward radiation was rather small, because there wasn’t much of the sourcing GHG above it. But after the addition, there is a lot more GHG above it, so the downward flux is now greater than it was before. One way of thinking about it is as a bounce of the formerly escaping IR photons against the new layer of GHG. So there is now an increased radiation flux downward, which will heat up both the lower atmosphere and also the ground directly (if the optical depth between the photosphere and the ground is not too great).
3) In the examination of the model for the GHE above, the initial radiation balance, plus the adiabatic-lapse rate, is what has set the structure of the temperature profile; and then the addition of more GHG to the temperature field causes a radiative imbalance that changes the temperature profile until the imbalance goes away. In the event of an atmosphere devoid of absorption/emission lines, the same strategy applies, but with different results: The temperature profile will be set by the radiation balance, with the “naked planet” temperature at ground level; convection/latent-heat considerations means that the adiabatic lapse rate will still apply, so that the atmosphere is colder than the ground. During the nighttime, however, the colder atmosphere will provide no insulation and no blanket.
Tom, Neal
Thank you for your responses, some useful insight on the GHG free atmosphere there: at night such a system must cool very quickly, like the Sahara desert – on speed 😉 .
Neal, on point 2, I understand the concept at a high level, Im just not clear that it has actually been proven to exist in reality. The reason Im lairy of it is that I dont see how a radiative equilibrium that doesnt actually occur (it must be some sort of average over a time period?) could actually be a mechanism to raise the temperature of the planet? It seems backwards (reminds me of Spencers recent paper about clouds). Also is it just the GHGs emitting? In our GHG free hypothetical system the atmosphere would still heat up in the day, and would therefore still have to rid itself of the energy by radiation? And if its not just the GHGs emitting, does that have some impact on the concept of IR opacity raising the altitude of the photosphere? I suppose some numbers would help, like increase of CO2 by 100 ppm, increases IR opacity by blah, raises photosphere altitude by blah. And then have these proposed effects been measured. Sorry if these are stupid questions.
I don’t see how making the discussion more technical and nuanced will add to the public’s understanding of the issue. In general, the public is bored with nuance and technicalities, so I’m not sure your complaint makes much sense. As far as the public goes, they want a simplified explanation. Online resources and op-ed writers talking about WV as the “most important greenhouse gas” or “CO2 is only 0.033% of the atmosphere” are misleading simplifications, and I don’t think the IPCC explanation is misleading at all.
The other problem is: how far do you want the IPCC to go? For instance, should they talk about the IR absorption from such molecules as N2?
I agree that citations or a “further reading” section would be helpful and interesting. Maybe that’s a comment for AR5.
#7
Steve M’s gripe is MY gripe:
In energy-producing States there is a fair amount of skepticism, healthy and unhealthy. How am I supposed to talk to them about the likely impacts of climate change on ecosystems if the basic mechanism underlying it all is portrayed in such a cartoonish manner? The IPCC cartoon has hurt me more than it has helped.
RE: #17 – The “public” includes non climate science scientists and engineerings, as well as math heads, econometricians, statisticians and other analytical types. By failing to present real math, the IPCC gives the impression they either don’t know the math, or, don’t want other math literate people critiquing it.
“engineerings” > engineers. Dyslexia plus fat fingers plus lack of coffee. 😆
Re: #3
As I pondered your motivation for laying out what the IPCC has presented in explaining the greenhouse effects, I was thinking that perhaps posters’ comments had some basis in it. I judge that a careful reading of what you have presented in this series of threads on the topic could go a long way in at least referencing a knowledge base from which to expand to the more technical expositions for which you are making a case.
I think that the question that is not addressed by the IPCC explanations that might be better spelled out in exposition form is what are the specific and detailed assumptions made in these calcualtions and what uncertainties do they contain. The IPCC explanations, for the radiative transfer processes, as I see them, are probably noncontroversial amongst the nearly all the scientists involved in these fields, but the main story is in the details.
While the warming due to 2XCO2 without feedback usually always comes out to 1.2 degrees C, when the feedbacks are included the warming varies by model from very little additional warming to several degrees more. Thus one would expect that that the warming models without feedback are better established and agreed to than those with feedback. That does not mean that the public, and particulaly the technically inclined public, would not be interested in more details on all aspects of the climate modeling results.
One must consider that the IPCC is rather obviously promoting immediate mitigating actions by governments of the world and doing this almost requires that they avoid exposing the public (that the IPCC are attempting to convince) to issues that might place uncertainties in their minds either by direct revelations or by making the subject seem complicated and difficult to pare down to simple answers.
Once such an IPCC exposition of the assumptions, complications and uncertainties of climate models was constructed and made public, it would immediately have to lead, in my view, to more questions from the informed public such as what does calculating a mean global temperature change mean to individuals who have to deal with local conditions and not a global average and what are the assumptions, complications and uncertainties that the models contain when it comes to determining the detrimental and beneficial effects of a “global” warming in localized areas of the globe.
I think that one must also look to how the participants of the IPCC are polictically motivated and how that could explain their hesitancy to provide an exposition. I would estimate that a very significant majority of the scientific participants are politically disposed to thinking that mitigating actions will be beneficial no matter what the extent of climate change turns out to be. They also are inclined to integrate the whole of the evidence for AGW as advocates for mitigation and be less likely to dwell on the uncertainties of separate issues. When a show of hands is required to address uncertainties on separate climate issues I think the integrated view remains very much in play.
#15, Gary Moran:
– Well, the photosphere is well-defined, not a time-average. The total flux incoming from the sun and outgoing from the ground&atmosphere can be regarded as either a time-averaged 1-dimensional or a spatially averaged 3-dimensional calculation, but I don’t see it as a problem either way: you can figure out if a bathtub is going to overflow by average input minus output, even if the rates are non-constant.
– For the photons of interest, it is only the GHGs that are absorbing/emitting: if gas molecules don’t have quantum transitions with the right energy differences, they can’t interact with the photons.
– In the GHG-free system, the local atmospheric temperature would follow the ground-level temperature, with the adiabatic lapse rate determining the temperature profile. It would be constrained by convective activity to keep in step with ground-level temperature, not by radiation.
– The other questions are not applicable: gas molecules can only interact with photons with which they have quantum transitions (including the continuous-band quantum transitions, not just the line spectra). The transitions available to non-GHG gas molecules are not in the IR, at the energy level appropriate for thermal relevance.
If the ghg-free (i.e. totally transparent) case is convection limited, the high-ghg case would be too; i.e. there would be more radiative transport, not less. Are you sure you want to say that?
#29, Larry:
Indeed, in the case of GHGs, convection is operating: That is why we have the adiabatic lapse rate. As described, the whole temperature profile gives rise to the radiative transport, which gives rise to the heating at ground level, which gradually raises the whole temperature profile (via convection) until radiative balance is achieved.
See #14.
>> in the case of GHGs, convection is operating
Actually, Larry said that it was “convection limited”, not that convection wasn’t operating.
30, 32, Gunnar is correct. Neil, you seem to have a real reading comprehension problem. This is at least the third time you got something that I said completely wrong. Slow down and make sure you understand what people are saying.
#33, #32:
What do you mean by “convection limited”?
Could those who believe in man-made global warming answer a simple question?
Shouldn’t it be warmer already?
For example: Central England Temperatures
Just a few to think about:
Year – Average Temperature
2007 – 10.48C
1995 – 10.52
1990 – 10.63
1959 – 10.48
1949 – 10.62
1921 – 10.47
1834 – 10.47
1733 – 10.47
http://www.metoffice.gov.uk/research/hadleycentre/CR_data/Daily/HadCET_act.txt
.01C above 1733,1834 and 1921 i [snip – please do not editorialize]
The problem is not that the IPCC doesn’t fully explain how global warming actually works – how global warming actually leads to a 2.5C temperature increase, the problem is that noone seems to have done so.
36, to use your words, convection-limited would mean “It would be constrained by convective activity to keep in step with ground-level temperature, not by radiation.” I think the reason why Gunnar understood that at face value is that limiting is a basic concept in electrical engineering that also applies to heat transfer; if one mode is responsible for more transport, it’s limiting. In the troposphere, convection seems to be determining the rate of heat transport. Above the troposphere, radiation governs. If there were no greenhouse gases, it would be less clear whether convection or radiation governed in the troposphere, since there would be a lot less resistance to radiative transport.
11, EGHE, LWR
Since I just had to look it up: http://climateaudit101.wikispot.org/Glossary_of_Acronyms#L
“Longwave (infrared) “greenhouse” radiation”
Shameless plug for the acronym list. I’ve be upgrading it with links to the organizations, projects and concepts in CS’s alphabet soup. Boy, there are a lot of them!
Contributions welcome.
Cheers — Pete Tillman
ClimateAudit 101 team
http://climateaudit101.wikispot.org
or to put in non scientific terms: an athlete has trained for muscle strength only. Then, during the event, he was cardio-vascular limited.
You can put racing tires on a chevy mailibu, but in a race with race cars, you would be engine limited.
If almost all of the heat transfer from surface to upper atmosphere is by convection, then you can change anything you want about the radiation system, more GHG, less, it won’t matter. The system is convection limited.
Re Tom Vonk, 12 ; Gary Moran #11 zero-order modelling
Gary Moran #11:
“3. 33′C effect of GHE: seems like a strange argument. The real comparison would not be to an airless planet, but one with an atmosphere of just oxygen and nitrogen. The impression I get is that we should infer such a system, because the atmosphere would be transparent to LWR, would behave like a planet without an atmosphere; whereas surely it would behave as I mentioned in my first question: with convection dominating; I would expect such an atmosphere would still moderate the surface temperatures in the day by cooling through convection, and insulate at night; and of course this atmosphere would still be capable of radiating away its excess energy. To me this is a fundamental comparison that should be modeled, and I find it bizarre that its never mentioned and instead we are pointed at a totally meaningless comparison instead, why?”
Tom VonK: your second remark is an interesting one . I have also been looking everywhere for a simulation of a planet with non IR active atmosphere (f.ex nitrogen only) . Unfortunately that doesnt seem to have been done .
Indeed such a planet would probably be very violent .
The night half would cool extremely fast by radiating everything towards 0 K while the day half would heat fast too .
The daily min/max differentials would be very large …
============ end quote ============
Are there any projects planned or in the pipeline to do basic climatology studies on Mars? Must be something, as there have been news reports about Martian GW. Again, empirical studies (imo) are what’s needed to make progress in climatology — the modelling is fine, but you aren’t going to discover interesting new stuff by modelling. GIGO….
TIA & Cheers — PT
Re 37, let me clarify something. If the two processes are in parallel (such as convection and radiation in the troposphere), the stronger effect is limiting. If they are in series (such as the different layers of the atmosphere), the weaker effect is limiting. This is all basic series/parallel impedance stuff in EE, but applies to heat transfer, as well.
#37, Larry:
OK, I think I understand what you and Gunnar may be getting at:
– In the GHG case, the escape of IR is limited by radiative transfer, in the sense that RT sets a cap for escape from the photosphere. The transport of energy to the photosphere is by both convection and RT.
– If there were no GHGs, there would be no cap on the RT, so I guess you could say that there would be more RT in that sense, although it’s RT that is completely de-coupled from the gas through which it is passing. The result is that, yes, ground-level nighttime cooling would be due solely to RT.
And I change my mind about convection in this GHG-free case: I have to remember that the adiabatic lapse rate only sets an upper limit of the temperature decline with altitude; in fact conditions of temperature inversion, in which the temperature is constant or rises with altitude, are also stable. So, there is no real “need” for the temperature to decline with height. I guess there would be no troposphere, in this alternate-world atmosphere? Seems odd; but now that I think about it, this issue also come up in a discussion with lucia.
A problem in communication on these topics is that I haven’t had the responsibility of presenting this formally, so it’s not going to be a well-thought-out explanation: I have to do interpretation of my understanding of how the equations work, on the spot.
#42, Larry: (This post wasn’t in view when I was doing #43)
So, adopting/adapting your terminology:
In the GHG case, RT and C are in parallel up to the tropopause, but it’s RT alone in the stratosphere.
So RT in series with (RT // C); RT at the photosphere is the limiting issue.
In the non-GHG case, RT is in parallel with C, but there’s essentially no “resistance” to RT, so C is short-circuited => C is a non-issue.
44, that’s how I’d draw out the impedance network, but in the case of no ghg, I don’t think that we can know in advance whether radiation of convection is the major mode in the troposphere, without doing the calculation. There’s resistance to radiative heat transfer even in a vacuum.
Let me take that a step further. Convective heat transfer generally lumps all specifics into a heat transfer coefficient h, and then works by Q = h(DT). To compare that to radiative, you have to convert (T2^4-T1^4) into that form. If you factor, you end up with (T2-T1)(T2+T1)(T2^2+T1^2). You have to basically assume that (T2+T1)(T2^2+T1^2) is constant for small changes, and generate a pseudo heat transfer coefficient. Then convection and radiation are in similar form.
This is supposedly a plot of the transmissivity of pyrex. You already new it was transparent to the visible 400 nm to 700 nm.
( http://www.valleydesign.com/Pyrex.jpg)
Larry: “0.6 out of 33 is ‘greatly intensified’?” That’s the point, it’s not, even if it accurately reflects anything physical.
Jae: It’s the same sort of effect, holding in warmth. Simple though, yes. As far as water vapor in the tropics, they even say ” In the humid equatorial regions, where there is so much water vapour in the air that the greenhouse effect is very large, adding a small additional amount of CO2 or water vapour has only a small direct impact on downward infrared radiation.”
Boris: “should they talk about the IR absorbtion from such molecules as N2″ Yes. It certainly seems to me they are trying to imply that CO2 is all that’s involved.
Gunnar, Andrew, Neal, Jae, etc….. If something holds in heat, it holds it in. Who cares if it’s by internal energy, phase change, radiation, convection or conduction? The point is there’s a limit to things and stuff moves around.
Compare two 40×40 greenhouses, one totally made out of 1/2″ thick clear glass where the outside temperature is -20C and one with transparent 1/20″ plastic on top and hollow 2 foot wide cinderblocks where the outside temperature is +40C and the ground covered with 1/2” thick plastic in both. Allow for 10,000 cu ft/hr air transfer. Three experiments; spray both with 100 gallons of water. Temp after 2 days? Let dry. Spray #1 with 100 gallons and #2 with 10. Temp after 2 days? Let dry. Reverse the amount of water. Temp after two days? Now redo those three experiments with the reverse outside temperature for each greenhouse. Then repeat those two sets with a 50,000 cu ft/hr air transfer. Then repeat those four groups of 3, but this time fill the greenhouses with plants (of all the same kind, a random variety).
Know what you’ll end up with after the 24 situations? I have no idea either. Why don’t we model it.
John Lang: “how global warming actually leads to a 2.5C temperature increase, the problem is that noone seems to have done so.” They can’t explain how CO2 does that, that’s why. Even if you isolate it from everything, you can’t, much less in the real world.
@Larry–
I think the confusion is people switching between 1-d and 2 to 3 d thinking. We had a similar discussion on another thread, and Neil is pretty much saying what I said. It’s a discussion that is based on a 1-d model or earth. (It’s useful to think of the 1-d before the rest. This is true even though the things you are thinking about are real and happen in the real 3 D earth.) But, Neil is totally correct for the 1-d earth
Pretend the earth can be approximated by a 1-d model for earth, where the earth is treated like an isothermal body (say a nice copper sphere) with some sort of atmoshpere. The temperature in the atmosphere is allowed to vary with elevation, but that’s it. (That’s the 1-d).
Suppose there are GHG’s, the earth radiates. The atmosphere absorbs energy and then re emits. We’ll call some level of the atmosphere the tropopause.
1) Neglect convection and conduction, and do the radiation problem, you will get a particular temperature profile. (It will be wrong, but forget about that for now.) The surface of the earth will be warmer than the tropopause. For all practical purposes, the tropopause is the location where the earth emits. (It turns out the prediction for the earths temperature is will be way too high.)
2) Next, in the 1-d model, do a very simple convection adjustment. Where the temperature gradient in the atmosphere is too large, just recognize that’s unstable, force heat transfer rate that will impose the highest possible stable lapse rate. March forward in time, and you get a new temperature profile. The tropopause is still colder than the surface, but the temperature difference is smaller than predicted when you neglected convections. (The temperature of the tropoause is the same as in the last problem, it’s the surface of the earth that’s cooler. This method gets closer to the right answer.)
This is the normal way to a 1-D radiative convective model to find an approximate solution for the effect of GHG’s.
Now, remember you did step 1? Now, assume the level of green house gases drop, so the atmosphere becomes more and more transparent. Redo the problem. As you redo the problem, you’ll find the surface of the earth predicted in step 1 drops as the atmohsphere becomes more transparent. In the limit where the atmosphere is totally transparent, the tropopause and earth have the same temperature. (The surface is a cold frozen snowball.)
This is because there is no resistance to radiation. So, temperature gradients are zero. There is no need to do step 2. If you did it, you’d find the temperature gradients are still zero.
This is because when the atmophere is tranparent, radiation presents zero resistance. In a 1-D model, the temperature of the earth matches the temperature of the tropopause. Convection doesn’t matter.
You may now recognize the problems with the 1-D model and proceed to the 3D model where one side of the planet is currently heated, the other is not and solve a horrible transient problem as the earth spins.
I’m guessing the air rises at the poles. But also, wind will tend to come around from the cold side to the hot side. You will need to deal with heat capacity, and do loads of other stuff.
But as far as EGHG goes, the 1-D cartoon does describe some trends.
Lucia,
That’s the mistake I made at first. That’s not true. In a complete vacuum, there’s a finite heat transfer rate. There’s “resistance” that’s a property of the surface emissivity and of the S-B constant. So the “resistance” doesn’t go to zero, and it’s a horse race between convection and radiation even in a transparent atmosphere.
#44, #45: Larry
– I’m not going to follow you down this road: I don’t see that making analogies between radiative-transfer issues and linear electrical network elements helps at all. Heat conduction satisfies a linear equation, but radiative transfer does not. I have never heard of anyone use the term “impedance” in connection with radiative transfer, unless they were talking about the impedance to the electromagnetic field, of which the radiation power is a quadratic (not linear) function; and I see no reason to innovate in that direction myself.
– When you use T^4 as part of the transport equations, you are basing that on the Stefan-Boltzmann law, which is about the radiant power integrated over all frequencies. That is really not relevant to the discussion on the real argument for GHE & EHGE, which depends on frequency-specific issues like the absorption coefficient. I know that this approach is taken for the hand-waving argument we saw much earlier – but that is one aspect of why that is merely a back-of-the-envelope calculation, not a real explanation of the GHE.
52, If you’re saying that this kind of hand-wave isn’t rigorous, and you can get a better answer by modeling from first principles, that’s obvious. But then you can’t make any qualitative judgments about sensitivities. Generally, I think Lucia laid the whole thought experiment out well in 49, and that give both the path to a model and the conceptual groundwork for sensitivity discussion. The one nitpick that I had in 50 notwithstanding, I hope you can agree with Lucia, because 49 is about as good as it gets without going into pages with illustrations.
RE:52
For what its worth, electrical analogies are used quite extensively in acoustics.
#49: lucia
I basically agree with your presentation, with one nit to pick: The effective point of radiation is the “photosphere”, not the tropopause.
54, electrical analogies are used quite extensively in heat transfer, too, but radiative heat transfer doesn’t fit neatly. Such are the transport analogies.
Heat transfer is one of the three transport process described in the seminal work (I’m sure Lucia will recognize this) “Transport Phenomena”, by Bird, Stewart, and Lightfoot (1957, IIRC). The other two are mass transport (i.e. diffusion) and momentum transport (from which the notorious Navier-Stokes equations are derived). Mass and momentum transport have no analog to radiation, either. But then again mass, momentum and heat transfer have no analog to inductance, otherwise electric charge transport would be the fourth transport process.
Radiative transfer can be linearized so that it fits into a model of an impedances plus a voltage bias. The analogous term to impedance would be obtained by differentiating Stephan Boltzmans law with respect to a change in flux. The analogy to voltage would be the temperature at which Stephan Boltzmanns law was linearized. The analogous term to current would be the change in outward power flux from the equilibrium.
#50, Larry:
Sorry, I can’t agree with you that there is finite heat transfer when there are no GHGs (completely transparent atmosphere). In that case, the gas and the radiation don’t affect each other at all, so the temperature appropriate to the radiation doesn’t change as it progresses through the atmosphere. Looking at the RT equation (other thread), it amounts to having no sourcing at all, so the intensity of the beam doesn’t change.
#53, Larry:
The back-of-the-envelope calculation is completely other from what I am interested to explain, and is not necessary to take the model discussed so far to a conclusion on the numbers (expected to be 3.7 W/m^2 for a 2X in C-O2), and the sensitivity (omitting all feedbacks). Instead, what we need are:
1) A model for the density of the atmosphere, as a function of height. Fairly straightforward in 1-d.
2) A calculation of the optical path (integral of the absorption coefficient) as a function of frequency and height. This seems straightforward, although I would prefer to find out more about the topic of saturation from a real book on RT.
3) Modification of 1) with 2X C-O2, well-mixed.
4) Modification of 2) with 2X C-O2, well-mixed.
1) & 2) describe the normal GHE; 3 & 4) describe the EHGE.
For 1) & 2), determine the parameters such that the total outgoing energy (integrated over all frequencies) gives the total incoming energy. This integral can be done at the OD = 1 point, because that is the photosphere radius for the IR, and for the non-GHG frequencies it doesn’t matter where you do the integral.
For 1) & 2), determine what the immediate difference is between the temperature of the old photosphere and of the new photosphere. The difference between the two is how much the ground-level will have to rise to shut down the radiative imbalance. And that is, I believe, what was wanted.
#57, John Creighton:
Many things can be linearized, in some approximation, for some regime. What impact will doing this have on the range of application of the model? I don’t know.
But I just don’t see how doing so will make anything easier.
#59 Over the range which temperature various on earth Stephan Boltzmans law is pretty linear. The point of the electrical analog is not to make things easier. What it is, is a way of visualizing how various feedbacks interact with each other.
#60, John Creighton:
But the Stefan-Boltzmann law is just not applicable. Why use it?
#61 Neal, I´ve read many times that Stephan Boltzmann’s law is quite accepted and non controversial. The application of the law though would be more doubtfully. To get the right answer the power output via the law must be reduced some how, either by selecting the right feedbacks or applying it at some hight above the surface where we arrive at the right answer.
However, maybe Stephan’s Boltzmann’s law should be more controversial as it doesn’t apply at the thermosphere and according to the following:
http://www.climateaudit.org/?p=2562
one could reason that the tropapause is not a suitable place to apply the law. Thus Boltzmann’s law can give you a good answer as to the outward radiation in the lower atmosphere but perhaps radiation is not the dominate cause of the net energy flow out of the lower atmosphere. This would render the Boltzmann law useless.
@Neil– If you say it’s the photosphere. I’ll go with that. Larry, Pat and Gunnar know I’m a continuum mechanics gal. If it’s not a continuum, I know nothing about it.
@Larry– The outline I discussed is actually in a book called “A Climate Model Primer” but I do need to check a bit. The discussion does work unti the optical thickness == 1. I may need to do more (and read up on the photosphere ) to nail stuff down.
Yes. I recognize Bird Stewart & Lightfoot, and am happy to tell Neil that the expansion you discussed is extremely useful in applications for mixed radiation convection. There are many 1-d problems were we all discuss conductances, resistances and linearize radiation and convection, because we have ot. (I’m sure Neil has done a Taylor series expansion and dropped terms in his lifetime too.)
That said, I think we are all going to have to draw our little cartoons and figure out which expansions really make sense here. (That is, if we really think the tranparent atmosphere is something we want to understand! )
Neil in #58– the bit responding to #53 particualry after “what we need are”. Yes. 🙂
FWIW. I’m willing to post things that have a high risk of being totally wrong, with the goal of having people correct me and eventually get it right. I’m willing to even post wrong things by people using pseudonyms who don’t want to be embarrased that thing are wrong. Then, we can all correct them and get them correct and do over.
This would sort of be like “The climate change black board”. (Maybe that’s what I should name my blog!)
#62, John Creighton:
The Stefan-Boltzmann law is inapplicable because it describes the total radiated power from a black (or grey) body, integrated over all frequencies. Such a body has spectral density given by the Planck distribution. It cannot have any gaps or emission lines in it’s spectrum.
But the GHE depends critically on what is happening at a specific frequency band, the “greenhouse” band. Using the SB law to handle this situation is like using the budget of the State of California for guidance on your household spending; or trying to understand molecular dynamics in terms of the Navier-Stokes equation.
The SB law is perfectly true, but, in this context, perfectly useless.
#63, lucia: The Blackboard
That’s probably a good idea. It would be possible to sketch out a fuller version of the list of #58, to break it into sections. Then start to fill in.
Several appendices for technical background that is not part of the main flow of the argument.
Good information on the spectral lines.
Fully fleshed out, I think the paper would run to 10-15 pages. Heck, we could probably get it published in the American Journal of Physics, since there’s no clean exposition anywhere.
>> electrical analogies are used quite extensively in heat transfer, too, but radiative heat transfer doesnt fit neatly
interesting sidenote: In this context, it’s an analogy. However, radiation is electromagnetic radiation
>> The difference between the two is how much the ground-level will have to rise to shut down the radiative imbalance. And that is, I believe, what was wanted.
1) Have to? There is nothing in nature that wants to balance it. Picture a pool filling up with water. There is nothing in nature that wants the level to stay the same. Water will rise until it flows over the side. It flows over the side only because that’s where the pool ends, not because nature prefers the level to stay constant.
In a similar way outgoing radiation is controlled only by the current local temperature of the effective outward facing atmosphere. If the situation is such that the incoming energy doesn’t cause that area of the atmosphere to heat up, it won’t balance. It doesn’t have to. The internal energy of the earth just went up. There is nothing in nature that wants to keep the internal energy constant.
2) We know nothing about the time period between when a chunk of energy comes into the system and when it goes out. It could be one hour, one day, one year, one millenium.
3) Your statement above assumes that the shape of the temperature gradiant cannot change. I see no physical reason why it can’t. If I’m right about that, the surface can stay the same temperature, and the temp gradiant curve can change (representing the stratospheric C02 being heated by outgoing IR). The surface is the warmest, everything above it is cooler. Soccer balls don’t roll up hill.
Hi Neal, I have a question.
I was wondering what the limiting affect on the optical depth is as the CO2 concentration increases in the photosphere? You say that where the OD = 1 point, which represents the radius of the photosphere for IR, and as the concentration of CO2 increases, this radius shifts upwards. Correct me if I have mischaracterized what you have been saying. I am wondering what impact gravity has on this phenomenon. As we increase CO2, the surface warms, more water vapour, more warming and so forth. With all that CO2 and water vapour, i.e., mass, there is likely an important gravimetric influence at some point with respect to the OD = 1 point photosphere radius?
I think Gunnar may have asked you this question somewhere, if so, please direct me to that link.
#56, Gunnar:
– Electromagnetic radiation power is quadratic in the field strength. So linearity, which applies to the field, isn’t going to help much.
– Have to: In the sense of a boundary condition: All that energy will be pouring in from the sun, and suddenly less of it will be escaping. That condition cannot go on: when the internal energy of the Earth goes up, the temperature goes up. What were we talking about again?
– Time taken: A chunk of energy is not a well-defined concept. Energy is fungible, so “duration of residence” doesn’t mean anything.
– Shape of profile: To reach steady-state, the temperature at the new higher photosphere must attain the same temperature as was formerly at the old photosphere. If the ground-level temperature remains the same, then the steady-state temperature difference will be the same as before, but with a longer distance, so the temperature lapse rate would be less than the adiabatic lapse rate. While there are certainly times that this is true, I somehow doubt that it will remain this way forever (which is what would have to happen): like a permanent temperature inversion. It doesn’t happen now, why should it happen then? I suspect that someone who knows more than I do about meteorology can give a simple explanation.
#57, Ian McLeod:
No, the OD(r) is just the integral of the absorption coefficient, at the frequency of interest, from r = infinity downward towards r = 0. It depends on the density and temperature of the GHG, not on anything gravitational.
I don’t know what you mean by a “limiting” effect.
Okay, the OD( r ) has some value depending on the density and temperature of the GHG, or better stated the solution of the integral of the adsorption coefficient. What I was trying to ask, was as the density of the GHGs increase, does Earth’s gravity play a roll affecting the solution of the absorption coefficient integral? At some GHG density, does gravity become non-trivial and limit OD( r )?
Don’t bother answering my last question, it is dumb. I am interested if gravity plays a roll concerning the GHE as water vapour and CO2 concentration increases. At least how you have described the GHE over the past week or so.
#61, Ian McLeod:
I can’t see how it would do so.
OD is basically the integral of the absorption coefficient (which depends on the quantum structure of the molecule, and the effects of temperature, and density) over distance.
I don’t see how gravity can work into that.
>> That condition cannot go on
Sure it can. Jupiter is in a state of profound radiative imbalance. I think it’s been that way since the solar system formed, with no end in sight. If you really think about 1st law, you’ll see that it only reduces to radiative balance if internal energy remains constant, and work is zero. But there is nothing in nature that wants to keep internal energy constant. IOW, nature has no problem with things heating up and cooling down. Please read my 1) again.
>> when the internal energy of the Earth goes up, the temperature goes up.
But not necessarily the temperature of the effective outward facing atmosphere. It could do some more work, build a tree, or heat up internal parts.
>> so duration of residence doesnt mean anything.
You missed my point. You get to radiative balance only when you integrate time from zero to infinity. When will Jupiter be balanced?
>> To reach steady-state, the temperature at the new higher photosphere must attain the same temperature as was formerly at the old photosphere.
You imply that you have a physical law here. Changing towards steady state requires a delta T between one mass and another. There is no physical law that stretches across time.
>> I somehow doubt .. I suspect that someone
That doesn’t give me a warm fuzzy.
>> Shape of profile … temperature lapse rate …
I’m not aware of the concept “temperature lapse rate”. But to make some sense of your longer distance, I suppose that little bits of C02, warmed by absorbing outgoing IR, thermalize that energy to adjacent atoms, then rise to get the longer distance. That wouldn’t heat the surface. The shape of the temperature gradiant graph simply changes.
#63, Gunnar:
– I’m not on Jupiter, which is called a “gas giant” because it’s physical structure is completely unlike Earth’s, and has all sorts of energy-producing reactions going on that are definitely not happening on Earth. On Earth, we don’t believe that the terrestrial temperature will climb and climb and climb and climb and…, without some reason. But if you’re willing to credit that, why get hung up on a measely 2.5-C degree of AGW? It’s like swallowing a porcupine but choking on a grape.
– I don’t believe that adding a bit of C-O2 to the atmosphere will suddenly mean that the adiabatic lapse rate is no longer relevant. It’s like finding out that merchants will suddenly no longer accept your credit card when it has only reached 83% of the credit limit, even though the bank swears your credit limit hasn’t changed. It doesn’t make sense to my physical intuition; I’ll discover an explanation for it later. I’m not a meteorologist, so I’m not aware of all the fine points of atmospheric behavior, but I can spot a weak point in a physical explanation, and that kind of weird behavior sticks out.
Because the policy wonks and alarmists are proposing shoving said porcupine up our arses to save us from death by grape.
Mark
Neal J. King #21
Im not sure what you mean here, an atmosphere without GHGs must still rid itself of heat, and the only option is radiation. Are you saying that is not happening? If so what would be your alternative explanation? Thanks.
#66, Gary Moran:
In #34, I argued in fact that an atmosphere without GHGs (i.e., with no possible way to interact with radiation) has no need to rid itself of heat.
Please look at that.
Neal J. King #67
An atmosphere without GHGs will still warm by conduction (contact with the surface) and will then distribute that heat by convection; so it will warm up, and will need to rid itself of heat, and that mechanism will be radiation.
#68, Gary Moran:
Why will it need to get rid of heat?
Our atmosphere does, because the Earth has to get rid of influx of solar energy and it does this by radiating IR; in order for that to work, the atmosphere must get colder with height, or the photons won’t be able to get through and out.
But if the atmosphere does not interact with the IR, the Earth will get rid of its heat directly via photons, unhindered by the atmosphere. Just like the hot stratosphere: It just stays hot.
[It should be understood, when I talk about “needs” of the Earth, that I am not projecting an intelligence on anything: What is needed is that certain boundary conditions and conservation laws be met. And what I am claiming is that, if the gas interacts not at all with radiation in the IR, that there is no particular boundary condition or conservation law that requires that the atmosphere gets rid of heat. This whole topic is a bit weird, in the arena of alternate-world physics.
I think I’ll sleep on it.]
Neal J. King #69
you need to re-read what I wrote. Absorption of radiation is not the only way heat is transfered from one medium to another.
Re # 40 Lucia
Maybe not. Remember that the geothermal gradient is from 5 to 40 deg C per km depth and although its response is slow (rocks are efficient insulators) the globe is still generating internal heat that is escaping via the surface. Some estimates put the watt per sq m value so low that it is trivial compared to other heating, but at least we know that it is a real effect and we know its sign and we are sure that it will continue for longer times than GCMs. You have only to go down 100 m in some locations to get a temp change the same size as AGW predictions.
I think everyone is just too hung up on GHGs. What about the other 99+ percent of the atmosphere. Remember that the N2 and O2 has to become heated and cooled every day, too, and this involves a great deal of energy gain and loss. It can easily be demonstrated, in fact, that it takes all the energy received in a day to warm an air column about 5 km deep by about 15 C at the surface, tapering to 0 C at about 5 km. Could the GHG effect be simply a heat storage phenomenon?
Geoff, #71. I recently did the calc. for geothermal heat, with numbers from CRC handbook; for heat=(~45 X 10^12) Watts, area=(510.072 X 10^6)km^2 =8.223 X 10^-2 watts/sq.meter. CRC also references heating by subsurface depth at 30deg./km.
jae, #72. I have wondered about that too. If we are talking ppm, and consider a million marbles, it is hard to imagine how changing 100 of these marbles will have some short-term (decades) miraculously large effect on not only the other 999,900, but the surface of the land and oceans.
This is one excellent theory. My view is that Gaia’s moist convection, the source of all life, is ever-healing. Basically, Gaia loves babies. If Steve M would provide a thread for that topic I would be happy to expand in factual depth.
Gary Moran #68
You are of course right with the exception of the word “need” .
The atmosphere doesn’t need to get rid of heat , it radiates because it does like every matter does as long as the temperature is not absolute 0 .
As it radiates , it looses energy .
And as it looses energy , it looses temperature .
It is irrelevant if that matter is iron , sand , nitrogen , water or CO2 .
Sometimes coming back to some simple basics helps – radiation are electromagnetical waves .
Any moving charge creates electromagnetical waves and as any matter is constituted of moving/vibrating charges , any matter radiates and looses energy through it .
Absorption/emission phenomenons are just frequency dependent interaction modes between matter and radiation that superpose on the above all present radiation (by analogy think resonant modes) .
So our GHG free atmosphere would radiate all it must and loose energy by that process exactly in the same way as a GHG full atmosphere does .
The only difference would be that as we assume that both the planet surface and the atmosphere radiate in IR , there would be no interaction between the radiation and the matter within the atmosphere .
But there would still be gradients , convection and conduction within the GHG free atmosphere because without it the whole atmosphere would be fast at 0K as it radiates to the empty space while the ground is at whatever equilibrium temperature it should be .
Is a resistor a capacitor?
Tom Vonk #75
In which case, does the oxygen and nitrogen in the earth’s atmosphere emit significant amounts of radiation (at night for example)? if so how does it interact with the atmosphere, and does it have any bearing on the GHE?
75, in reality, with a low-density gas in a fairly unconfined space (such as the atmosphere) natural convection heat transfer will always be much greater than conduction. Conduction only enters the picture in the films that contact solids.
Gary Moran #77
Of course they do and not only at night . They have a temperature different from 0 , haven’t they ? So they radiate .
At the temperature the atmosphere is , it radiates infrared so this radiation interacts with the atmospheric matter exactly in the same way as the radiation emitted by the ground does . A molecule doesn’t care if the origin of the electromagnetical wave it is interacting with
was a SiO2 crystal on the ground or a N2 molecule .
And yes it has a bearing on the GHE .
I have written somewhere a rather long post about the quantum interactions in the lower atmosphere but don’t remeber where .
Basically the energy absorbed by the GHG (f.ex the 15µ radiation for CO2) is not reemitted because the relaxation time is too long .
Now as we are in LTE , the quantum energy state repartition follows the Boltzmann distribution ands must stay constant .
For CO2 at such low temperatures most molecules are in the fundamental state and the few that are vibrationnaly excited are mostly in the 15µ bending mode (because it’s triple degenerate) .
So now the newly excited CO2 molecules must deexcite because else the density of the 15µ state would violate the Boltzmann distribution .
The way they do it is through collisions .
As there is only N2 and O2 around them , they transfer the absorbed energy to N2 , O2 molecules (symetrically it works also the other way round , they get vibrationnaly excited by collisions too) .
And as energy can’t accumulate , it is finally radiated out by N2 and O2 but of course no more as the “original” 15µ radiation .
On top of that , N2 and O2 absorb and emit themselves because of the colisionnaly induced dipolar momentum but that works only for IR below some 500 cm^-1 .
All this is no more true above some 50 km because there the LTE doesn’t hold anymore .
That’s why the calculation of the 3,7 W/m² of “radiative forcing” is really not an easy matter to be derived .
No, but who says air is just a resistor? How about an R-C circuit?
Can radiative transfer be modeled as a network? The first inkling I have is a store and forward network.
79: Tom Vonk: Thanks, I think you have just explained “thermalization” to me.
#71, Geoff Sherrington, #73 Steve Keohane: Geothermal energy
As Steve Keohane already demonstrated, the contribution of geothermal energy to the heat flux is negligible.
#72, jae, #73 Steve Keohane: Other gases and heat storage
The distinction of GHGs among all gases is that they affect the rate of the cooling capability, in a way that non-GHGs cannot. A crude analogy: There’s a whole lot of mass to a bathtub, but only two bits of it affect the water level: the plug and the tap (and only the angle-setting of the tap). The GHGs are sitting at a very high-leverage position in the power budget.
#79, Tom Vonk:
Interesting.
#70, Gary Moran, #75, Tom Vonk: Heat conduction & GHG-free atmospheres
I think we (including me) are getting a bit wrapped around the axle concerning what would happen in the case of a GHG-free atmosphere: after all, there is no such thing. However, the real issue is, What is the role of non-GHG gases in the real atmosphere? Because in reality, all these gases have absorption lines somewhere in the spectrum, so they all interact with radiation at various frequencies. GHGs are special because they have lines within the IR bands that fit into the remaining spectral “windows”; but then why are those windows important, when the non-windows have been closed exactly by absorption by other gases?
A preliminary thought is that it may also have to do with the fact that the temperatures of interest (around -18 C) are high enough to generate these IR photons. But I need to think about it for a day or so. I may not respond to anything for awhile….
#79 Tom Vonk:
Doesn’t Kirchhoff’s radiation law require that in a mixture of gasses in thermal equilibrium with the walls of an enclosing container, that each molecule must reradiate a quantum for each quantum absorbed? I really think that if it didn’t apply I could build a perpetual motion machine.
Neal J.King # 83
As for me I don’t really feel wrapped around anything 🙂
As for your question , well the role of the non GHG gases is nothing less than fundamental for the radiation transfer .
After all they make for some 99 % of the molecules .
So some 99 % of what the atmosphere radiates is coming from them .
However when the question is what interacts most with mid IR radiation then despite their 99 % , it is not them .
So they dominate on the emission side but not on the interaction/absorption side .
What about a capacitor and two inductors?
There’s no heat transfer analog to an inductor. However, with enough capacitance (heat capacity), you could in theory get a reactive effect to diurnal cycling, contributing to overall impedance. Surely one of the climate scientists has explored that.
Tom Vonk posts are 100% correct
How about IR hitting molecules and causing vibration/bending/stretching etc which is then re-emitted in various other ways? Nothing at all like a magnetic field expanding and contracting as current flow waxes and wanes (day and night, solar variation, etc)? How about the climate system as one big LC choke-input filter smoothing out sunlight in and re-emssions of the land out and the interactions of the GH and non-GH gasses?
A calculation which I have looked for, and been unable to find, is a calculation of the efficiency of gas molecules absorbing and then emitting radiation. Nothing has 100% efficiency. A molecule that absorbs radiation and then emits radiation will do so with either a change in the amplitude or the frequency of the radiation. Does anyone have a reference to an equation for calculating this?
I would just like to know how much of the IR energy that is absorbed by the GHG molecules ends up in thermalizing the N2 and O2 that makes up about 98+ percent of the atmosphere. All this gas has to be heated (and cooled) each day (diurnal variation). You can easily use up 11 kwh/m^3/day energy to heat a 5 km x 1 m^2 column of air by 15 C at the bottom which decreases linearly to 0 C at the top. Is there enough energy “left over” for all this radiative transfer to take place?
GHE is often explained with mechanisms that seem, to me at least, unphysical. E.g. radiative balance, increased atmospheric opacity to IR, back radiated etc. I’ve been pondering this, and I think it might be because if the effect is considered radiatively then it gives a handle on which to try and work with the problem, which otherwise wouldn’t be there. For instance it is often said that if there is less outgoing radiation than incoming (another way of phrasing the IR opacity model?), then the system much adjust to balance this and so the temperature at the surface rises. Personally I have a few problems with this explanation: there is no law of conservation of radiated energy to force this, and it sounds suspiciously like a perpetuum mobile; however like I’ve said in previous posts if this is really an effect of IR that would otherwise escape being thermalised (is that a real word?) rather than a cause (like Spencer’s paper on cloud feedbacks, here on CA a few weeks ago), then it makes a bit more sense; I mean in terms of what really happens it would be false, but at least you could understand where they were coming from. It’s like some strange climatology radiative double speak. It would also explain why these IPCC primary level explanations and diagrams seem so weird. And also why there are so many different explanations of the GHE (many listed in the G&T paper).
Just musings really.
Neal J.King # 83: I think it’s useful to understand that a lot of heat is being moved around without the GHGs and that it’s not just them emitting; some diagrams (cartoons?) of the GHE have CO2 almost acting like a reflector of IR radiation. I’ve found Tom’s clarifications very useful.
@Gary 92:
The principle is called conservation of energy.
Tom Vonk:
We’ve had this discussion before elsewhere. Nitrogen interacts with IR in the 5 to 100 micrometer band only by formation of collisional dimers. The binding energy for these dimers is quite low so their lifetime is quite short. The extinction coefficient for absorption is also 5 or 6 orders of magnitude smaller than that for water vapor in the same wavelength range. The absorption is also continuum, not line. If the collisional de-excitation time of excited CO2 is too short for emission, how can a highly unstable nitrogen dimer possibly have time to emit? Besides, there are orders of magnitude more elastic collisions than inelastic, so in fact the lifetime of an excited CO2 molecule before an inelastic collision is much longer than the one nanosecond average time between elastic collision. That means the emission probability for excited CO2 (and water vapor) is significantly greater than zero and explains the observed emission spectra which show strong CO2 (and water vapor) emission spectral features rather than the continuum emission that would be expected if nitrogen emission dominated.
jae says:
January 9th, 2008 at 10:09 am
Yes with a time constant of a few hours as demonstrated by the lag in temperature w.r.t. diurnal variations. ( hottest part of day lags maximum insolation by about two hours or 30 degrees).
DeWitt Payne says:
January 9th, 2008 at 6:08 pm
It really depends where you look the atmospheric gases N2 and O2 absorb and emit from far IR to radio frequency bands. RSS and UAH satellites measure radiation from O2 in 60 GHz to determine troposphere temperature. Hence while the energy emitted for each wavelength might be low is the area under the very broad curve over the very wide spectrum that gives us the total emission and makes it significant.
Here is a link to a Spectral Calculator which covers all the gases which might be in the atmosphere.
Click on Spectral Database Browser – then Atmosphere Browser – set the wavenumber to 0 to 3,000 which covers the majority of the visible and far infrared spectrum and you can compare the absorption bands of all the various gases. The Y axis gives the efficiency of absorption
CO2, for example is about 100 times more efficient at absorbing infrared emissions at the 600 wavenumber (15 micron) level than H2O. You might need to know a little about the physics to be able to use it.
http://www.spectralcalc.com/spectralcalc.php
Quote:
What about a capacitor and two inductors?
I think Sam wants to have his “PI” and eat it too…
No doubt about that, but what happens after the CO2 absorbs that IR? Does it give it to all the N2 and O2 molecules during collisions to “even out” the temperature in the atmosphere, or does it reirradiate it all up and down and sideways? Or both? That N2 and O2 have to come up to ambient temperature somehow.
Lucia #93
Yes but that applies to all energy transfers, not just radiative. As a pretty poor example photosynthesis will lock in radiative energy from sunlight, the energy hasn’t been lost it just isn’t radiative anymore. Of course in the broad brushes we are talking about I’d doubt that photosynthesis would be detectable, but it demonstrates the principle
Jan Pompe #96
That was sort of what I was alluding to with Neal, but didn’t have the physics knowledge to backup: if the GHGs aren’t the only players in the absorption and emission game then the IR opacity model may have a few leaks? I’m afraid my maths and physics aren’t up to scratch on this, for me this is really a fishing exercise.
#100, Gary Moran:
It astonishes me that you are willing to promote the idea that a 2X in C-O2 will cause the Earth to increase in temperature indefinitely, while being unwilling to accept the possibility that it will lead to a finite temperature increase.
Likewise: I am trying to explicate what could reasonably be expected of the behavior of the system operating under known dynamics. It is always possible to ask, And what if there are unknown factors?
I don’t want to get Rumsfeldian on you, but come on…
LadyGrey #90
That calculation is called Quantum Mechanics 🙂
Each molecule and each energy transition has a complex valued function called transition momentum .
For instance the transition momentum from the ground state to the first excited bending state of CO2 by absorption of 15µ radiation is [PSI(ground) | µ | PSI (1)] where µ is the dipolar momentum .
This transition momentum allows to calculate the probability of the said transition called Einstein coefficient .
So for every molecule there is a set of Einstein coefficients whose number is equal to the number of possible transitions (and it is huge) .
There are 3 processes :
– absorption
– stimulated emission (basis of the laser effect)
– spontaneous emission
The Einstein’s coefficients for absorption and stimulated emisson are equal , the one for spontaneous emission is different .
Those coefficients are property of the molecule so don’t depend on equilibrium considerations and such .
On top of that and regardless of any absorption/emission properties there is thermal radiation that is only due to the fact that charges that compose the molecules accelerate by vibration and collision and this property is general for any form of matter .
This radiation is continuous and its intensity depends on many things like temperature , pressure etc and the frequency distribution is black body like .
In the case here , the absorption probability of 15µ radiation by CO2 is very high .
Follows that the stimulated emission probability is also very high .
However at low temperatures as I have already said only a small number of excited states exist so the gaz is dominated by absorption and not stimulated emission .
The probability for spontaneous emission is much lower .
Therefore at low temperatures and high pressures as is the case in the low atmosphere , the equilibrium between the different quantum states (the proportions must stay constant) is mainly ruled by collisions .
The transfer of energy happens here by inelastic collisions between the excited CO2 molecules and other molecules .
The probability of such a collision is called inelastic cross section and can again be calculated by QM .
Here it is however more complicated because there is no need that the quantum transmitted by collision be equal to the absorbed quantum and the cross sections depend on energy .
Now the observation of the spectrum and the mean time between collisions shows that the spontaneous emission hardly happens .
F.ex if the spontaneous emission was the dominating process , you would see about half of the 15µ radiation emitted by the ground going up (the spontaneous emission being isotropic , half of the emission goes up and half goes down) .
What you see instead is an almost complete extinction in the low atmosphere .
As this absorbed energy can’t disappear (like Lucia reminded , this mystery is called energy conservation :)) , it has been reradiated at different frequencies and that is exactly what the collisionnal deexcitation does .
>> It astonishes me that you are willing to promote the idea that a 2X in C-O2 will cause the Earth to increase in temperature indefinitely, while being unwilling to accept the possibility that it will lead to a finite temperature increase.
Neal, I’m not seeing where you get to this from what Gary wrote in #100. Gary is just correcting the description of the 1st law, since 1st law doesn’t apply to one form of energy alone.
Neal J. King #101
Obviously this is a bit of a leg pull, however I’m willing to play along in the cause of elucidation. I’m not suggesting that an imbalance between incoming and outgoing radiation in the earth won’t, over time, reach a new equilibrium. However there is no law that says radiative transfers have to balance, in fact we know from the law of conservation of energy that this isn’t the case: a solar panel has no radiative equilibrium because the incoming radiation is converted into heat. Therefore in a situation where the incoming radiation is greater than the outgoing, this must be a symptom of a change in the system (i.e. outgoing radiation is being converted to heat) and isn’t the mechanism that actually instigates the change. Hope this makes sense.
And I do accept that the GHG’s in our atmosphere change it’s thermal characteristics, I do accept that the GHE exists. My main skepticism centers around the climate sensitivity. However I’ve recently become very intrigued by the official explanations of the GHE, which, as I’ve now repeated quite a few times, seem unusual. I’m trying to understand why it is explained in these terms, and I’m also a bit suspicious of the motives! Anyone who seen a Realclimate thread weave endlessly for weeks on end simply because the team absolutely insist that back radiation does heat the surface, even though a more careful use of words would have closed the inquiry off very quickly, may understand where I’m coming from.
Tom Vonk: Do you have an estimate of 2 X CO2 sensitivity?
@Gary– Draw a control volume, and apply conservation of energy. When accounting for the rate of change of internal energy, feel free to account for photosynthesis converting thermal and radiant energy to heat on. But if you do that, don’t forget to account for releases of energy, like plant matter decaying and rotting, plant and animal metabolism, forest fires, human industry an the like which reconverts that energy into heat. What with old logs rotting in the woods, and my cat racing around the living room, I suspect you’ll find the net effect of plant and animal life on this calculation very small.
The net effect may be small, but isn’t it what has given us stores of carbonates and hydrocarbons, over long periods of time?
================================
Jae # 105
No .
I believe that it should be positive but can’t offer any solid argument by how much . I also believe that this effect is overwhelmed by non linear interactions among the multidecadal events (ENSO , NAO etc) that appear chaotic and impact strongly the climate at time scales generally considered (decades to a century) .
Besides I strongly oppose (like R.Pielke and many others) the idea that the “global time average of the surface temperature” has any physical meaning or is a valid metrics to measure the “climate” and I can’t see the beginning of a valid reason why it should correlate to any relevant dynamical parameter .
Consequently I think that the “sensibility” defined as the variation of an unphysical parameter following a modification in the radiation transfer processes is an uninteresting non sense .
As for the so called “radiative forcing” , I believe that the often quoted value of around 3 W/m² may be a right order of magnitude .
However this is only a radiative argument and the atmosphere does many other things than only radiating .
It is impossible to know what this figure would give in a realistic moving , scattering and humid atmosphere without running a whole convection/radiation model what I have of course not done .
The only caveat I would say is that the incertitudes or outright ignorance of more important processes (clouds , particules , precipitation , land use ) is of the same or bigger order of magnitude than the already mentionned 3W/m² .
For anecdote : Dan Hughes has once looked for the energy dissipation in the boundary layer and in oceans because that is never taken in account .
It seems that very little research has been done on that but that it might be around 1 W/m² +/- something .
One could say that it stays constant but most people know what mess a non zero constant can do in a nonlinear system where it was previously set to 0 .
Also it is not clear if it would stay very constant if the climat changed .
In any case there is preciously little about this subject .
Gary Moran says:
January 10th, 2008 at 2:20 am
There is a hole in the bucket
dear Henry dear Henry
A hole
Sorry about that but it leaks like a proverbial sieve. While the atmosphere is opaque at certain frequencies the energy absorbed is shared with the other gases in the atmosphere through collision this will warm the atmosphere more than it would be through conduction and convection alone. This energy is carried aloft by convection where the remainder of the atmosphere is essentially transparent to the emitted radiation largely because there is next to no water vapour, which is the larger absorber, in the stratosphere and above.
>> I suspect youll find the net effect of plant and animal life on this calculation very small.
lucia, Gary said it was probably undetectable, so not sure why you made this point.
However, I would like to point out the contradiction between your statement above and the AGW speculative idea. If the entire plant and animal kingdom are so insignificant, then surely, so are small changes to a trace gas. There is certainly more empirical evidence for the effect of the plant and animal kingdoms than there is for the temperature effects of C02.
As I’ve previously shown, C02 doesn’t have enough mass to have a big effect. That’s why AGW explanations subtly imply that C02 reflects radiation. Yet, no one is willing to say that out loud, since it’s impossible. I think that’s what Gary means when he says that the official explanations are “unusual”.
#110 Gunnar
Of course the animal and plant life have insignificant effect but artificial trees will have a significant effect.
@Kim
Yes. But if someone is going to account for the associated heat storage effect on the planets temperature, they also need to account for the current rate of release.
If an effect of photosynthesis is small in a particular heat transfer problem, then compared to other effects, it can be ignored. But what you can do is suggest it’s large and then only account for the heat sinks, and but forget the heat sources.
If Gary wants to account for the heat sink to photosynthesis, he needs to account for the heat source do you animal life, the rate at which we are burning fossil fuel, and even, quite possibly, heat generated in nuclear reactors.
I may well be wrong, but I suspect if he does a back of the envelope calculation, he will find all effects are small when we look at the heat balance between the planet and the universe.
My main point is: He can’t just suggest it’s large, arm wave at the heat sink (photo synthesis) and forget about the heat sources (plant and animal metabolism, industrial heat generation.)
@Gunnar–
Sure Gary suggested the effect admitted it was small, and claimed that, as a broad brush issue, we need to be aware of the effect. In reality, as a broadbrush issue, phenomena that are identified and can easily be shown to have no no detectable effect can be neglected.
So, if we assume Gary’s major point is his claim about needing to think about these picky insignificant details when we are taking a broad brush view, he is wrong. If we can show they make no difference to the answer, and everyone agrees the effect is miniscule is so, we can all proceed to agree to ignore them.
We do not need to account for my cat taking a poop and shifting the litter in his box when estimating the effect of GHG’s on the earths climate.
As to your suggestion that my comments about photosynthesis contradict the possibility that CO2 affects radiation, the short answer is: You are wrong. The Greenhouse gas doesn’t not involve the heat capacity of CO2, and so the mass fraction of C02 in the atmosphere has nothing to do with this.
I won’t discuss this further except to note that we have discussed this repeatedly on unthreaded. The explanation involves thermo. Thermo is banned. In particular, theories involving gross mis-applications of thermo are banned here. (I totally understand why SteveM does this.) I’m not going to invest the time addressing your heat-capacity of CO2 theories and write paragraphs on this here just to have them deleted.
Eventually, I will post about that theory at my blog.
Lucia #112
Sorry, I think you are misunderstanding where I was coming from. I’m not saying we should account for photosynthesis, just that an imbalance between incoming and outgoing is not a mechanism, but an effect, and I was using that example to illustrate the point.
#104, Gary Moran:
– I think you mean that the solar panel doesn’t balance radiatively because it produces electricity, not heat. If it only got hot, it would indeed rise to the temperature at which, between IR radiation and heat conduction, the in-flow would equal the out-flow.
– But in the case of the Earth, where is the “electricity” (the non-thermal store of energy)? and there’s nothing to conduct the heat to, either. Growing plants and stuff doesn’t seem to be on an ongoing increase in terms of amount: things seem to be pretty stable. Where would all that extra energy be going?
Inquiring minds want to know!
Neal J. King #115
Again, I’m just saying there is no law of conservation of radiative energy, so it can’t be a mechanism. If something occurs to cause an imbalance between incoming and outgoing radiation, then you can’t, as a matter of principle, say that the system will change to maintain radiative balance, it depends on the system. With a solar panel it aint happening. With the earth we would expect balance once equilibrium has occurred. I think it is important to understand what is cause, and what is effect.
Gary– 144.
But, ok, what is your point? That we can’t apply the first law of thermo? That we can never even in the simplest, zero order, models for climate, neglect miniscule effects to get a first approximation.
More complex models exist, and they effects other than the change in the dry bulb temperature when estimating the rate of change in the internal energy of the planet. So, if your large point is that models should include these sorts of effects, the answer is: they do. They include the ones that matter: latent heat of vaporization of water, heat of fusion of ice, etc.
Look, I think GCM’s are approximate; I doubt they are better at predicting the change in Temperature due to a doubling of CO2. But there is no point in saying they are mistaken because they make assumptions they don’t make!
@Neil
The energy is all wasted by teenage boys pondering impure thoughts.
Please don’t go all Rumsfeldian on us. Donald Rumsfeld fired my Dad in the great Searle flushout of 1978!
I think you’re way wrong here, Steve. What you’re overlooking is the need for explaining the science at multiple levels so that different kinds of people can understand. There’s no question that the main text should be presented at the full-bore scientific level. But it’s important that somebody present the material at a level more understandable to the nontechnical reader.
Now, at this point we run into a serious problem: anything less than the whole truth is a lie. Every teacher must lie to the students to help them reach the point where they can understand the whole truth. And who is to decide the shape of the lie? There’s political content in every simplification. Nevertheless, it is possible to make a good faith effort in that direction, and I believe that the IPCC text does represent a good faith effort to present a simplified model of the truth.
I’ll also remind you that the general public’s appreciation of the issues is well below the level of presentation of the IPCC simplifications. In my interactions with people about climate change, I find that very very few understand the basic principles at ANY level. The IPCC explanation, if read and understood, would take people a long long ways towards understanding.
Your standards are unrealistically high. The perfect is the enemy of the good. We need to get people to understand really basic concepts before they can understand advanced concepts. The IPCC explanation seems excellent to me.
Chris, I hope you don’t invest your money in schemes that have documentation as poor as IPCC’s.
Chris Crawford, I find on the contrary that this is the crux of the philosophical problem here. “anything less than the whole truth is a lie”… perhaps it wouldn’t be so if the debate had remained at the scientific level. But in the hurry to have the question settled, IPCC science has involved many advocates on the basis of over simplified statements while still being presented as the ultimate truth by its members (chapter one: IPCC, chapter two: IPCC etc…).
But regardless of this unfortunate stray into sensationalism, we all know that some problems (scientific, legal or artistic) require to go through the looking glass of appearences before key elements surface and an understanding emerges. For instance, many music work reflect this search of a defining moment, where you feel the whole paradigm does go through the hole of the needle and emerges altered forever. Although not a lawyer, I have been explained that this is indeed in a complex case this very search, of the details, that will find the angle to prove or disprove a case. So indeed I find your plea for labelling the search for knowledge “unrealistic standard” extremely self serving and philosophically dead wrong: why not stop research and pack now then. Are you concerned about the reputation of IPCC and followers in the mind of Joe Sixpack or, to paraphrase Prof. Marcel Leroux, about figuring out why some regions are warming and others are cooling?
I am interested in the latter hence my support here.
No, I want to have my P=IE and eat it too.
>> The Greenhouse gas doesnt not involve the heat capacity of CO2, and so the mass fraction of C02 in the atmosphere has nothing to do with this. I wont discuss this further except to note that we have discussed this repeatedly on unthreaded.
lucia, why are you getting tense? Actually, it’s more basic than thermo. However, to set the record straight, it wasn’t discussed repeatedly, it was one time, and there were no serious challenges other than blanket assertions that the mass of C02 has nothing to do with the GHE. By that logic, increasing the mass of C02 by human emission shouldn’t change the effect.
You should know by now that thermo is not banned, Steve just wanted it discussed in dedicated threads. These threads now exist. However, this is right on topic for this thread, which is about exactly how the GHE works.
I remember that several people responded to the blanket assertions against my argument, agreeing with me, saying “they knew of no physical theory that didn’t involve C02 absorbing energy”. Let’s try this thought experiment:
An enclosure is exposed to a strong IR source. We start with a single C02 molecule in the enclosure, measure the radiating W/m2 coming from C02. Increase C02 content until we get to 1 atmosphere of pure C02, measure w/m2. Does the w/m2 remain constant, regardless of the mass of the C02 in the enclosure?
Re #32, Martian climatology
About the only thing vaguely substantial I found in Googling was Habibullo Abdussamatov’s speculations: http://news.nationalgeographic.com/news/2007/02/070228-mars-warming.html
He hasn’t published this — or, indeed, anything that looks substantial per Google Scholar. He does, however, get several pers. comm. cites in Z. Jaworski’s immortal ”CO 2: The Greatest Scientific Scandal of Our Time” 😉
Anything else substantial around re Martian climatology?
Incidentally, this is another illustration of Google’s failure to index CA properly: if you Google “basic climatology studies on Mars” , an exact phrase from my post 32, you will get… nothing. Very unsatisfactory.
Pete Tillman
#83, me: on non-GHGs
I’m going to return to the scene of the crime.
Last time, when we were arguing about whether (non-existent) GHG-free gases would be convective or not, it bothered me that I didn’t have a clear-cut explanation of why some gases are GHG and others are not: After all, they all have absorption lines somewhere, and leave their mark on the solar spectrum. So I took off a little time to think about it.
My conclusion is the following: Using the hand-waving argument we all know and distrust, based on the naked-Earth model with no spectral break-out, we conclude that the surface of the Earth ought to be around 255 K. That means that the Earth will be emitting most of its thermal radiation in the wavelengths in the general neighborhood of kT/hc, which is 1.38-23 * 255/(6.626e-34 * 3e8) = 56.6 microns. Therefore, any absorption band within spitting distance of 57 microns should be taken into account with respect to radiative-transfer effects on the energy-transport question; anything very far away need not be. So we are talking about the IR region.
So what would happen if there were no molecules with lines in the IR band? It would still pick up heat from direct conduction, as Gary Moran has insisted; and it would not be correct to say that there would be NO interaction with radiation (another point by Tom Vonk): if there are lower-energy bands, they will be used by the gas to absorb photons. So there’s no reason to think that convection will be missing from this picture. (I retract my conclusion in #69, and that particular point in #49.)
So one way to think of the cooling problem is by dividing the spectrum into many independent bands, which provide parallel cooling capabilities to the Earth. If a band is free of lines, that cooling capacity is unhindered; but if it has important absorption lines, it will be “blocked” => the same sort of photosphere questions we have discussed before: a high-altitude photosphere means little power can be transported through that band, whereas an unencumbered band has its photosphere at ground-level. The IR region is important because that’s where most of the Earth’s radiation’s energy is.
(I observe the analogy to what some people were describing as “linearizing the problem”. But I still don’t see any reason to adopt the terminology of “impedance”, etc.: I think it’s likely to suggest too many inapplicable approaches.)
#116, Gary Moran:
I’m not using radiative balance as a mechanism, but as the method of identifying where the mechanism will lead: the steady-state condition.
The mechanism I identified somewhere on this site as the increase in the downward flux of IR photons, which have kind of “bounced” off the added layer of C-O2 that was planted above the old photosphere to get it to the new photosphere (speaking sloppily).
>> But in the case of the Earth, where is the electricity (the non-thermal store of energy)?
The whole plant and animal kingdoms get all their energy from the sun. All the continuous movement of the air and water is also derived from solar energy. Besides, many things are not in radiative balance, from Jupiter to my house. What is it about the earth that is different?
>> and theres nothing to conduct the heat to, either.
Huh? If you look at the big blue marble from space, you’ll see that the atmosphere is a very thin layer. The earth’s crust and core are mostly metal, which is a very good conductor of heat. If the crust went up in temperature by a very small fraction of a degree, it would be the same as the atmosphere going up by a lot.
>> it would indeed rise to the temperature at which, between IR radiation and heat conduction, the in-flow would equal the out-flow.
Maybe eventually. But the point that I’m making (and I think Gary) is that there is no direct physical law that tries to balance it. You haven’t really understood or answered #58 and #63.
The key to understanding the error in the AGW speculative idea is to think about Jupiter (or my house). Neither are in radiative balance, yet 1st law is not violated. Jupiter receives only a little solar radiation, yet radiates out twice as much. There is no violation of 1st law though, because the error is not recognizing the other heat sources. Two other sources of heat are gravity (eg lapse rate) and a reservoir of hot material (core). Earth has both of these other heat sources as well.
All of this is not to say that AGW isn’t true, it’s just saying that the explanation provided doesn’t indicate that is.
re: M.Villeger, #121:
I think you misunderstand my point. I am not arguing that it is acceptable to lie in the less technical explanations; I am arguing that the process of teaching makes falsehoods inevitable. Therefore, the teacher must make a good faith effort to make his misleading statements politically neutral. Now, if you can offer an example of a politically biased simplification, please do so. Steve’s complaint was not that the less technical explanations are biased, it was that the less technical explanations aren’t technical enough. Perhaps he overestimates the scientific literacy of the audience. I don’t know.
>> Im not using radiative balance as a mechanism,
Then you would be the first AGW proponent that didn’t.
>> but as the method of identifying where the mechanism will lead: the steady-state condition.
We don’t know that earth was ever in this steady state condition. It could millions of years.
>> which have kind of bounced off the added layer of C-O2
Ahh, the reflection implication.
>> it was that the less technical explanations arent technical enough
Actually, his complaint was that there was no more technical explanation.
#127, Gunnar:
– Living stuff: Unless the amount of living stuff is on a secular increase or decrease, the energy tied up in living stuff is steady-state. It can’t serve as a constant drain. Conservation of energy applies.
– Jupiter: There’s a heck of a lot of chemistry going on in the Jovian atmosphere, I recall that the atmosphere itself generates heat. Are you suggesting the Earth is doing this too? Measurements don’t suggest it. The point is that the Earth doesn’t have a lot of ways to get rid of energy. Radiation is the main one; the GHE occurs when that is encumbered.
– “If the crust went up in temperature by a very small fraction of a degree, it would be the same as the atmosphere going up by a lot.” So now you suggest that the Earth’s surface is heating up, to take up the excess heat. Hello! That is EXACTLY what global warming is. So, yes it will do that, but it will also heat up the atmosphere to keep to the lapse rate, until the imbalance is fixed.
– Back to Jupiter: the planetary structure of Jupiter is so different from that of the rocky planets that Jupiter & it’s similar neighbor Saturn are called “gas giants”, in some ways something like stars in that their atmospheres producing power. Where do you see the Earth’s atmosphere producing power? Should an explanation of the GHE also be required to point out that the Earth is like a dog and does not run & carry thermometers to affect its temperature, as well?
#129:
– Are you paying attention to what I am saying, or are you trying to super-impose your understanding of what others have said upon it?
– Steady-state is always a question of time-scale. The steady state condition of a scoop of ice-cream over a 1-minute timescale is far different than over a 1-hour tmescale. If we were to slip in and mix well the extra C-O2 as in this intellectual experiment, the radiative-transfer situation would respond literally at the speed of light (divided by the appropriate index of refraction) and produce the radiative imbalance. The heating would start right away; coming to a steady state in terms of a stable temperature would take longer.
– The “bounce” I discussed somewhere among these threads as the interpretation for the increased downward flux that comes out of the RT equations. M.Simon raised this issue in an analogy to a ball of radioactive material which suddenly has an additional layer added to it, causing the inward neutron flux to suddenly increase. I had not thought of it myself until I tried to visualize how the changed boundary conditions at the position of the old photosphere had to affect the detailed dynamics of what was going on. (As I have mentioned before, RT is a little like hydrodynamics: Even when you figure out what it’s going to do, it takes some time to figure out how to explain why it’s doing that. Think about the Venturi effect.)
@Gunnar– Stop the accusations that my response to you are due to some sort of emotional state of mind. That’s a childish counter response.
Your general argument has been addressed before. There were plenty of counter arguments; you didn’t accept them. It was zambonied.
I have no issues about SteveM doing deleting; I totally understand. That doesn’t mean I’m want to spend time rediscussing all that.
As for your current thought experiment: If you think it can teach people something, answer your own question and tell us what you think it means. (That is: do it if you think SteveM doesn’t think you are starting to discuss your own idiosyncratic theory of something.)
Otherwise, I don’t immediately see any connection with that thought experiment and the subject of SteveM’s blog post. I don’t have any particular inclination to play 20 question simply to discover whatever explanitive theory you think you may have developed.
Lucia #117
I’m sorry Lucia we really are completely out of sync on this. The only point I’m trying to make around the term radiative balance, is in a similar vein to that Steve made about the IPCC FAQ, I believe it’s a poor explanation. This is from the FAQ:-
I don’t just think this explanation is grade school simple, I also think it suggests unphysical mechanisms. And I tend to find that the GHE is constantly described in ways that suggest the effect is radiative throughout, which I regard as a mis-characterization.
I agree with this from Gunnar
Neal J. King #126
Well my point was the IPCC explanation, if you don’t believe it’s a mechanism then we agree on something 😉
Gary, I’m not sure if you’ve ever taught physical science, but I have and I find the IPCC explanation quite clear. It’s a good first-order explanation. If I were to change it, I would change “much of this thermal radiation” to “some of this thermal radiation” and I’d add a sentence explaining that the back-radiation raises the temperature of the earth before being re-radiated. The failure to mention this is the biggest weakness of the explanation, but this is also the most difficult concept to communicate. Go ahead and try your hand at writing an explanation for this without using technical terminology. I think you’ll find it pretty tough. Here’s my best effort:
I would insert a new sentence prior to the last one saying: “This makes the earth a little warmer, which in turn increases the amount of infrared it radiates. The final temperature the earth attains represents a balance between the amount of radiation reflected back and the temperature increase due to that reflection of energy.”
Not very good, is it? I think you can see why they choose to leave it out. But I’d like to see your effort.
133, Gary Moran:
You focus on this statement:
I don’t read that as a description of a proposed mechanism.
The word “must”, used in this context, reminds me very much of someone trying to explain how he got the answer to a fairly complex problem: “I know this has to happen here, and that has to happen there; so (somehow) this other thing has to happen to bridge the gap in between.” They’re not filling in on the whole mechanism, because (as I mentioned before) the mechanism doesn’t, in fact, jump out of you from the equations: you have to probe innards of it a bit.
As Weart’s discussion of the history of the GW study indicates, the 2X issue has been the central problem in these studies, and a lot of people have stumbled across these concepts before. I would say it took about 3 generations of scientists to come to terms on it. But it’s much better a framework than you draw it out to be.
Gary– I think we simply disagree on at least one point.
You seem to be saying the first sentence in the first IPCC paragraph you quote is incorrect. Here’s the sentence:
I think that sentence is correct if we take the point of you with a control volume draw well outside the atmosphere. This is an application of the first law of thermodynamics, with a number of simplifying assumptions.
Radiation is the only heat transfer mechanism from that point of view. That’s literally true. There is no appreciable work done by the universe on the planet.
The only remaining question is whether or not the earth’s climate steady state. The planet isn’t literally at steady state, but for the purposes of estimating the magnitude of the impact of GHG’s, I don’t have any particular objections to assuming the climate is steady.
We use quasi-steady assumptions in engineering all the time, in circumstances with equally tenuous steady states. (We often say “quasi steady” in transport problems. Steady state simply doesn’t exist in many industrial process. Taken literally, steady state never exists for anything, anywhere. )
Anyway, I assumed your quibble was with the issue about assuming the earth’s climate is at steady state for the purposes of estimating the magnitude of the greenhouse effect, and that your photosynthesis exampled related to the issue of steady state.
But evidently, you and I both agree that photosynthesis makes no difference to that bit, and you have some other gripe with the assumption about that first sentence.
So, at this point, I’m left not knowing what you find incorrect about that specific sentence. Or, if that’s not the sentence that bothers you, I don’t know what is bothering you.
If your are only saying you think the documentation is poor, I agree. But that’s different.
I’d go very simple: Greenhouse gases in the atmosphere absorb heat radiation that would otherwise be lost to space, raising the average temperature of the system.
[snip]
However this line of thought is highly speculative, and I won’t spend any bandwidth defending it.
Chris Crawford wrote:
“I am not arguing that it is acceptable to lie in the less technical explanations;”
I never thought you did…
“I am arguing that the process of teaching makes falsehoods inevitable. Therefore, the teacher must make a good faith effort to make his misleading statements politically neutral. Now, if you can offer an example of a politically biased simplification, please do so.”
Of course agreed and that makes many schools reliance on some “inconvenient truth” very disturbing indeed…
“Steve’s complaint was not that the less technical explanations are biased, it was that the less technical explanations aren’t technical enough. Perhaps he overestimates the scientific literacy of the audience. I don’t know.”
Chris Crawford, I never wrote he thought they were biased. Since we are discussing the role of the IPCC and not the role of a grade 12 physics professor, Steve’s point was that even the most technical level achieved by IPCC -presented by many as the undisputable authority in the domain- fell short when scrutinized, a thorough process that is what science is all about. Thank you for allowing me to precise my point as surely we understand each other now.
Lucia #136
our disagreement is a very subtle one. Even though I agree the condition is likely correct: at equilibrium I would expect incoming and outgoing radiation to balance; it is not a mechanism involved in the GHE: if a radiative imbalance occurs it is a symptom of what is happening in the system. I also think radiative balance never really happens, the system is simply far too complex: day, night, seasons; but in general terms they effectively balance or a change in temperature must occur. From a modeling point of view it’s fine, but we are not talking about modeling, we are talking about explanations, an area where determination of cause and effect are important.
Hope this makes sense.
>> There were plenty of counter arguments; you didn’t accept them.
No, that’s not how it was. JohnV made the most vigorous counter arguments, and I worked him into a corner where he basically claimed that one could warm a swimming pool with a hair dryer. Your only argument was that a single molecule doesn’t have a temperature.
@lucia, I note well that you have suddenly become very evasive and dismissive. You are basically begging SteveM to zamboni my comments. Even if he does, it won’t change the fact that you can’t face the consequences of that simple thought experiment in #123.
Steve: Enough of this. Lucia, please don;t reply.
@Gary–
I think Neil already said it best. It’s true that particular radiation balance doesn’t explain the greenhouse effect. That’s just a heat balance.
Explainning the mechanism for the greenhouse requires discussion of radiation in the atmosphere itself. That’s where the “radiative/ convective” models, GPMs etc come in.
The IPCC does a poor job on those. These leaves the impressin that that little cartoon is the explanation. It’s not. All that cartoon shows is that there is something that even requires an explanation. That something is roughly 33K of warming.
Knowing something needs to be explained is an important step but it’s not an explanation.
Re: #108
Tom Vonk,
While I may disagree with you on some of the details of radiative energy transport in the atmosphere and the applicability of simple models, I am in general agreement with your views in this post.
On the subject of a planet with a completely transparent atmosphere: How fast the surface cools at night will be a function of the heat capacity of the surface and the atmosphere. I would expect the atmosphere to be a classical exponential atmosphere with pressure decreasing exponentially with altitude. The temperature lapse rate will depend on the initial conditions. If the planet and atmosphere were initially cold, then I would expect the lapse rate to be the dry adiabatic rate determined by the acceleration of the local gravitational field and the heat capacity of the gas. There would also be advective heat transfer (and probably Hadley Cells) from the equator to the poles. The diurnal temperature variation will depend strongly on the rotation rate and the heat capacity of the surface. In the limit of infinite heat capacity, there is no diurnal variation. At zero heat capacity the temperature will drop to the Cosmic Microwave Background temperature as soon as the sun goes below the horizon. but even an airless body like the moon has some surface heat capacity and the rate of temperature decrease will decrease rapidly with decreasing temperature and not nearly as far as the CMB (Stefan-Boltzmann does apply here). Also given that any real gas has some heat capacity, there will be some transfer of heat by conduction (and a resulting temperature inversion) to the surface at night. An atmosphere that is perfectly transparent to incoming and outgoing radiation cannot radiate and all its heat content comes from conduction from the surface and is transported through the atmosphere solely by convection with no loss of energy to space except for the tiny fraction of atoms at the top of the atmosphere that exceed escape velocity.
Gary Moran # 116
That is very true .
Why is understanding of radiation so difficult ?
Because radiation is not a vector field where you can simply calculate fluxes by doing scalar products .
An elementary surface on an infinite plane centered on M (x,y) radiates equally in all directions ,
there is no unique vector depending only on (x,y) and giving radiation in M .
Radiative “balances” always involve integrals over the whole space or simplified arguments by taking spheres large enough to be sure that everything that is not radiation stays within the sphere .
And even then it is not easy – look only how hard lived is that stupid argument that the GHE for the Earth represents 33°C that makes about as much sense as saying that a parabole is approximated by a horizontal line over the whole space .
Personnaly I find bottom up approaches of radiation (aka statistical thermodynamics & QM) better to lead to understanding .
Using that approach what can one say about an elementary volume dV of atmosphere containing Ni molecules of different species in LTE ?
It has kinetical energy (Ek) , potential energy (Ep) and internal energy (U) .
At the boundary it receives radiation Rr and it emits radiation Re . We can suppose that it doesnt exchange mass and momentum .
All molecules are distributed among the energy states according to Boltzmann distribution .
Now we suppose that what changes are the proportions of molecular species Ni in the volume .
What changes immediately is the energy states distribution that will lead to a new LTE so there will be a new equilibrium different by dU from the original .
If Rr is constant , everything will change so that :
dEk + dEp + dU + dRe = 0
And that’s all . Obviously there is no notion of “radiative balance” in there .
Now if the gaz we added has a property of presenting high energy states interacting with the received radiation we can say a bit more .
The energy state distribution will contain more of high energetic states .
That leads then to an increase of U .
If we suppose that dEk = 0 (there is no movement of the dV) and dEp = 0 (there is no vertical movement of dV) then dU = – dRe
As dU is positive , dRe is negative .
So one possible answer of the system is to increase it’s internal energy (temperature) and to radiate less what is very logical because Rr being constant , a bit more of it will be consumed to populate the new high energy states so less energy will be available for Re .
Of course it is not the only one possible answer .
It may do many other things with Ek , Ep and U depending on the system .
However what this simplified explanation does show is that radiation is not conserved .
Indeed if we had Rr = Re for all Ni (and T) , we’d have dRe = 0 and dU = 0 what is impossible because we need energy to populate the new high energy states and keep the molecules there .
For the sake of clarity – what I call here U is the sum of the classical internal energy (mean kinetical energy of the molecules) and the energy of all quantum states of the molecules . They both interact with each other .
Steve
If you want to go and do the full climatology course, and all the physics, go and do it. Stop whining that they don’t spoonfeed you whatever it is you want to know.
Steve: I don’t expect to be “spoonfed”; I expect organizations to do their job. I think that it’s reasonable to expect IPCC to provide a proper exposition of how the enhanced greenhouse effect works. If that involves some climatology, so be it.
Bugs says:
The IPCC has an obligation to explain itself fully if it expects governments to enact expensive public policies based on their claims. If climate scientists don’t want to ‘spoonfeed’ people with expertise in other scientific fields then they should go back into their labs and stop demanding radical social changes.
Erl Happ could tell me what soured your grapes, Bugs.
=================================
#144, Tom Vonk:
Nobody is taking it as a principle that radiation is conserved.
What is being conserved is energy: when the energy that leaves the Earth + atmosphere system is less than the energy that enters, per unit time, the amount of energy in that system must be increasing. For a simple system, the temperature will rise; for a complicated system, one would typically expect that average of the temperature over the system would rise.
This rise will continue until the rate of energy departure equals the rate of energy arrival.
So, finally, how does radiation get into this? Simply because the overwhelming proportion of energy received by the planet is by radiation, and the overwhelming proportion of energy departing the planet is by radiation. So in the (quasi-)steady state, there will be no radiative imbalance; and the system-averaged temperature will be higher than it was before.
Neal,
Your 147 is very good. I think we’re really making progress. So, with this better description of the hypothesis, it becomes clearer what data needs to be gathered to provide support for the idea.
Re: #143
If Rr is constant and dRe is negative and stays negative then the total system energy (Ek + Ep + U) will increase without limit. As this doesn’t (and can’t according to the Second Law) happen, there must be a mechanism that drives dRe back to zero as the system energy increases. That looks like radiative balance to me.
I think it’s the increase in the number of people listening to FM radio that’s causing all of this.
The IPCC reviews, as in scans the existing science as it is published, collects what has been learned from that science, summarises and publishes it.
It is not an educational institution. If you want to learn the basic science of climatology, do the university course.
Not to be too snarky about it, but Bugs’ quote would be more accurate if it read “The IPCC reviews, as in scans, the existing science that supports their conclusions as it is published, collects what has been learned from that science if it supports their goals, summarizes and publishes it.”
There is just too much science that never makes it into the IPCC reports to say otherwise.
Re: (hopefully not perpetuating an unwanted discussion):
[snip – nothing wrong with your reply except the above]
To paraphrase the IPCC description of the atmospheric greenhouse effect:
1. A warm body (the earth) radiates heat to a cool body (the atmosphere)
2. The cool body “back-radiates” (IPCC term) heat to the warm body.
3. This process continues perpetually, with heat flowing round in a continuous cycle.
4. The result of this perpetual process is that the warm body becomes warmer.
Gerlich has been published this year with a more rational view of this topic :
“Falsification Of The Atmospheric CO2 Greenhouse Effects Within The Frame Of Physics.” G. Gerlich, R. D. Tscheuschner
International Journal of Modern Physics B, Vol. 23, No. 3 (30 January 2009), 275-364
One Trackback
[…] I collated IPCC AR3 and AR4 “expositions” of the enhanced greenhouse effect, observing that, in my […]