Yesterday I collated IPCC AR3 and AR4 “expositions” of the enhanced greenhouse effect, observing that, in my opinion, they were so baby food as to be essentially useless to a scientist from another discipline. Today I’m going to drill a little deeper in the expositions, going to a 1995 journal comment by Houghton and to his text, Global Warming: the complete briefing, to see if either contains a more useful exposition. I’ll also comment on why I find the IPCC heuristic particularly unsatisfying.
Reviewing the bidding briefly, in AR4, I’ve been unable to locate any exposition of the mechanism of the enhanced greenhouse effect that rises above the following in the FAQ:
To balance the absorbed incoming energy, the Earth must, on average, radiate the same amount of energy back to space. Because the Earth is much colder than the Sun, it radiates at much longer wavelengths, primarily in the infrared part of the spectrum (see Figure 1). Much of this thermal radiation emitted by the land and ocean is absorbed by the atmosphere, including clouds, and reradiated back to Earth. This is called the greenhouse effect. … Adding more of a greenhouse gas, such as CO2, to the atmosphere intensifies the greenhouse effect, thus warming Earths climate.
I do not regard this as a satisfactory explanation, but note, as usual, that this does not mean that a satisfactory explanation cannot be provided, only that IPCC elected in AR4 not to provide one. I’ve not been able to locate any other attempts at exposition of the enhanced greenhouse effect in AR4.
AR3 was only a little a better: it mentions a heuristic for the mechanism as to how increased CO2 warms the surface as follows (1.3.1):
These so-called greenhouse gases absorb infrared radiation, emitted by the Earths surface, the atmosphere and clouds, except in a transparent part of the spectrum called the atmospheric window, as shown in Figure 1.2. They emit in turn infrared radiation in all directions including downward to the Earths surface. Thus greenhouse gases trap heat within the atmosphere. This mechanism is called the natural greenhouse effect. The net result is an upward transfer of infrared radiation from warmer levels near the Earths surface to colder levels at higher altitudes. The infrared radiation is effectively radiated back into space from an altitude with a temperature of, on average, -19°C, in balance with the incoming radiation, whereas the Earths surface is kept at a much higher temperature of on average 14°C. This effective emission temperature of -19°C corresponds in mid-latitudes with a height of approximately 5 km. Note that it is essential for the greenhouse effect that the temperature of the lower atmosphere is not constant (isothermal) but decreases with height. …
The increased concentration of greenhouse gases in the atmosphere enhances the absorption and emission of infrared radiation. The atmospheres opacity increases so that the altitude from which the Earths radiation is effectively emitted into space becomes higher. Because the temperature is lower at higher altitudes, less energy is emitted, causing a positive radiative forcing. This effect is called the enhanced greenhouse effect, which is discussed in detail in Chapter 6.
Unfortunately IPCC failed to deliver on its promise for a “detailed” discussion in Chapter 6, where the term “enhanced greenhouse effect” is nowhere in sight. In the TAR exposition, the requirement that temperature decrease with height is specifically mentioned, but they did not provide a diagram showing temperature changes in the atmosphere.
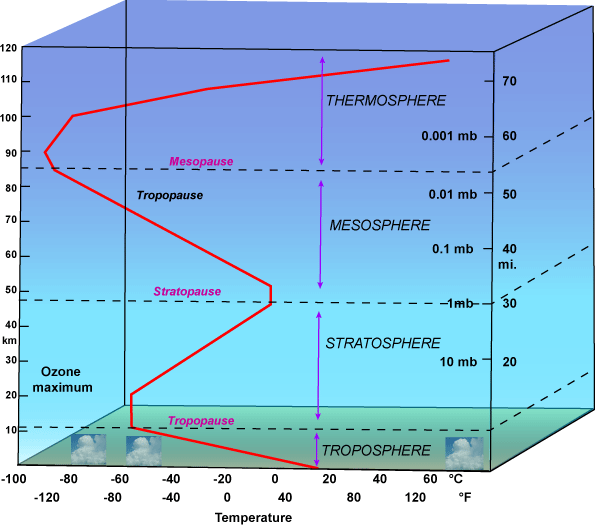
In fact, as shown in the diagram below, the atmospheric temperature profile is not a simple monotonic decrease in temperature as one might presume from the IPCC heuristic – the temperature decreases up to about 10 km (the tropopause) and then increases through the stratosphere (up to the stratopause). There are further interesting features in the very upper atmosphere which are not relevant here. The temperature increase in the stratosphere primarily results from the absorption of UV solar radiation from ozone.
Now it turns out – as I’ll discuss below – that a proportion of CO2 radiation to space occurs in the tropopause and a proportion actually occurs in the stratosphere. For the proportion in the stratosphere, the IPCC heuristic is reversed and increased CO2 would cause radiation-to-space in those lines to occur at a higher temperature, reversing the effect. Now the proportion of radiation-to-space from the stratosphere in practical terms appears to be very small and thus swamped by the tropospheric effect, but there’s no reason not to mention it in an exposition. The proportion of CO2 radiation from the tropopause is very large and important and should not have been passed over even in a heuristic.
Houghton (1995)
Let me now review some comments on this topic by Sir John Houghton, the IPCC WG1 Chairman, in 1995, in response to a comment by skeptic Jack Barrett in Spectrochimica Acta (1995) – a comment that was mentioned in passing in SAR. Houghton stated:
A further point that Barrett makes is to suggest that because most of the absorption by carbon dioxide from the surface occurs within 30 m of the surface, the enhanced greenhouse warming due to increase of carbon dioxide in the lower atmosphere is negligible. In fact, most of the enhanced greenhouse effect occurs not because of changed absorption of radiation from the surface (although some change does occur in the wings of the carbon dioxide band where absorption is weaker), but because as the concentration of carbon dioxide in the atmosphere increases, the average height (around 6 km) from which carbon dioxide emits radiation to space also increases. Since atmospheric temperature in the lower atmosphere falls with altitude, if nothing changes other than the amount of carbon dioxide, the amount of radiation to space is reduced. For atmospheric carbon dioxide, this reduction can be accurately calculated; for doubled atmospheric concentration it is about 4 wm-2. To restore the Earth’s balance the temperature throughout the lower atmosphere has to increase – hence the enhanced greenhouse effect [1- J.T. Houghton, Global Warming: the complete briefing. Lion Publishing, 1984.]
Note that there’s a discrepancy in the effective height between Houghton 1995 (around 6 km) and TAR (about 5 km), both heights safely below the tropopause at about 10 km. Houghton’s argument here (in the “peer reviewed literature”) does not rise above the cartoon discussion in IPCC TAR and AR4. However, he cites his textbook, which we now turn to for possible edification.
Houghton(1984), Global Warming: the complete briefing
At other wavelengths radiation from the surface is strongly absorbed by some of the gases present in the atmosphere, in particular by water vapor and carbon dioxide….Absorbing gases in the atmosphere absorb some of the radiation emitted by the Earth’s surface and in turn emit radiation to space. …
Radiation is emitted out to space by these gases from levels somewhere near the top of the atmosphere – typically between 5 and 10 k high (See Fig. 2.3) [Below is my re-plotting of Houghton’s Figure 2.3]. Here because of the convection processes mentioned earlier, the temperature is much colder – 30 to 50 deg C or so colder – than at the surface. Because the gases are so cold, they emit correspondingly less radiation. What these gases have done therefore is to absorb some of the radiation emitted by the Earth’s surface but then to emit much less radiation out to space. They have therefore acted as a radiation blanket over the surface(note that the outer surface of a blanket is colder than inside the blanket) and helped to keep it warmer than it would otherwise be. …
This increased amount of carbon dioxide is leading to global warming of the Earth’s surface because of its enhanced greenhouse effect. Let us imagine that the amount of CO2 in the atmosphere suddenly doubled, everything else remaining the same. What would happen to the numbers in the radiation budget? The solar radiation budget would not be affected. The greater amount of carbon dioxide in the atmosphere means that the thermal radiation emitted from it will originate on average from a higher and colder level than before (Fig 2.3). The thermal radiation budget will therefore be reduced , the amount of reduction being about 4 watts per sq meter.
One quick editorial point: to my knowledge, convection processes warm the upper atmosphere. So when Houghton says “because of the convection processes mentioned earlier, the temperature is much colder – 30 to 50 deg C or so colder”, I don’t get this at all – surely this is a mistake. But the most striking aspect of this particular reference is that it adds virtually nothing to the statement in Houghton (1995) or the cartoon in TAR. In each case, the result is stated, but not derived. I’m not saying that the argument is implausible – obviously it’s not implausible or else it wouldn’t have been relied on so widely. I’m only saying that all we see here are assertions, with no measurements or data. Houghton asserts 4 wm-2 as an impact number, but it just appears out of the blue.
Some Comments
A reader of these heuristics would conclude that CO2 radiation-to-space from the lower troposphere is the most distinctive aspect of CO2 radiation-to-space – radiation from about 5- 6 km. The next figure shows an upwelling spectrum from the Pacific (an image from the 1970s downloaded from John Daly’s site – a more up-to-date image should be around somewhere, but won’t change the main observations here):
The large notch or “funnel” in the spectrum is due to “high cold” emissions from tropopause CO2 in the main CO2 band. CO2 emissions (from the perspective of someone in space) are the coldest. (Sometimes you hear people say that there’s just a “little bit” of CO2 and therefore it can’t make any difference: but, obviously, there’s enough CO2 for it to be very prominent in these highly relevant spectra, so this particular argument is a total non-starter as far as I’m concerned. )
So when I look at spectra like this, I take away the impression that a very large proportion of CO2 radiation-to-space is from the tropopause (10 km) area. While a lot of radiation-to-space may come from 5-6 km altitude, the proportion of CO2 radiation-to-space coming from such altitudes looks like a relatively small proportion of the total. Because the temperature at the tropopause is more or less invariant with altitude, incremental CO2 will have a relatively small impact on any radiation within this funnel – which is the most important part of CO2 emissions. (You notice a little peak in the center of the funnel. The odd thing about this is that the center of the funnel is where CO2 absorption is the greatest and I wonder whether that might actually indicate some emissions from the stratosphere above the tropopause (but in any event, this is a very secondary feature.)
This seems very fundamental to me for the potential derivation of the logarithmic relationship. Most/all of the “explanations” of the logarithmic relationship volunteered here ignore the fact that the “forcing” depends on the atmospheric temperature profile – this is one of the reasons that I discourage these “bright ideas” unless the people have read the literature. The way that one would go about trying to establish a logarithmic relationship for additional CO2 around present levels is plausible would be to show that the combination of:
1) negligible forcing for CO2 radiation from the tropopause;
2) linear forcing for CO2 in the wings in the troposphere.
As temperature goes up, the “width” of the funnel would increase and the proportion of linear forcing to total forcing would decrease sort of as 1/n, yielding a logarithmic relationship. To some extent, that’s probably what’s done in the 1-D models, but the description of these models is very opaque and they don;t bother trying to explicate the mathematical properties of the model.
Now the Houghton back-of-the-envelope argument relies heavily on the fact that nothing else changes with additional CO2; it is my impression that this is also the case with Lacis et al 1981 and the 1-D studies. But it sure seems like a curious coincidence that the CO2 “funnel” just happens to be at the tropopause. Presumably the tropopause is where it is, at least in part because of CO2 radiation. Thus the whole premise of changing CO2 levels without changing the atmosphere temperature profile does not seem to me like a relevant method of modeling CO2 impacts, as higher CO2 levels would surely change the height of the tropopause and related atmospheric profiles. The assumptions on atmosphere temperature profiles intrigue and will be what I look at closely if and when I ever find a study that is on point to these issues.
References:
Houghton, J. Spectrochimica Acta A 51, 1391-1392.
J.T. Houghton, Global Warming: the complete briefing. Lion Publishing, 1984.






138 Comments
But it would be interesting to see a later spectrum chart, as it would show what the increase in CO2 (some amount, x, of change from 1970 to 2007) has done to the readings.
Since the AGW is assumed to be greater since 1975, this chart makes an interesting “baseline” (pre-warming).
The IPCC reports are not meant to be the science from first principles, and they aren’t going to put the bits in that you want just to suit you. They are meant for public consumption, and as such a comprehensive description of the science is way beyond the scope of the reports, and would have the general not reading them at all since they would not be able to understand any of the science.
Pierrehumbert has a partly completed textbook on the science on his home page.
http://geosci.uchicago.edu/~rtp1/ClimateBook/ClimateBook.html
Happy reading.
See the 2003 Science article by Santer and subsequent comments by Roger A. Pielke, Sr. and Thomas N. Chase for ‘height of tropopause and global warming’ exchange.
Whether the tropopause has increased in height 100m or so seems highly speculative and difficult to determine from my memory of reading these.
Seems to me that from an analytical perspective they are talking about some kind of partial derivative problem with boundary conditions. However, I have not seen it spelled out that way, and anyway, trying to assume the atmosphere has a ‘boundary’, or that there is an ‘average’ height has obvious hairs all over it.
Would also note that the phrasing around the lapse rate is very strange. The lapse rate emerges simply as a function of energetic considerations – mgh – so I have always found the discussion about changes to the lapse rate weird too.
Contributions of Anthropogenic and Natural Forcing to Recent Tropopause Height Changes
B. D. Santer,1* M. F. Wehner,2 T. M. L. Wigley,3 R. Sausen,4 G. A. Meehl,3 K. E. Taylor,1 C. Ammann,3 J. Arblaster,3 W. M. Washington,3 J. S. Boyle,1 W. Brüggemann
BTW I think this is a great line of enquiry and really captures my longstanding concerns in this area. I would like to see:
1. a mechanistic model of the AGW process no matter how imprecise, so the components could be refined. I think a similar thing has been done with the atmospheric reactions in the CFC case even though the coefficients were unknown to OOMs.
2. a real attempt by AGW supporters to find alternative explanations for ‘enhanced’ GW, to show it can be the ONLY viable explanation.
This is what you would see in any physics papers that are any good.
#3. David, re your second point, if increased CO2 causes forcing because of “the higher the colder”, one can easily envisage mathematics in which you had temperature increases localized in the upper atmosphere that more or less neutralized the effect for all practical purposes. Is that physically possible – even to some degree? When one reads about increased tropopause heights, it makes one wonder. When one broaches the topic, one gets arm-waving about the troposphere being “tightly coupled”. It probably is, but, as so often, one would like to see the details.
Doesn’t the surface area from which the IR is re-radiated to space also increase by a factor of 4pi if the altitude increases, such that while there may be less IR radiated per unit surface area, since there is more surface area, the total radiation wouldn’t drop as significantly as noted?
The following papers and references therein may help you. Chandrasekhar’s Radiative Transfer book may also be of interest.
T. Nakajima, M. Tsukamoto, Y. Tsushima, A. Numaguti and T. Kimura, Modeling of the radiative process in an atmospheric general circulation model, Appl Opt 39 (2000), pp. 4869–4878.
T. Nakajima and M. Tanaka, Algorithms for radiative intensity calculations in moderately thick atmospheres using a truncation approximation, JQSRT 40 (1988), pp. 51–69.
Not quite what you’re looking for, but Wearts http://www.aip.org/history/climate/co2.htm#S3 cites
Manabe, Syukuro, and Richard T. Wetherald (1967). “Thermal Equilibrium of the Atmosphere with a Given Distribution of Relative Humidity.” J. Atmospheric Sciences 24: 241-59 as “a calculation published by Princeton computer specialists in 1967: the first reasonably solid estimate of the global temperature change that was likely if the amount of CO2 in the atmosphere doubled.”
—-more on this below.
Ah, and an appropriate quote:
“No branch of atmospheric physics is more difficult than that dealing with radiation. This is not because we do not know the laws of radiation, but because of the difficulty of applying them to gases.” G.C. Simpson (1928), “Some Studies in Terrestrial Radiation.” Memoirs of the Royal Meteorological Society 2(16): 69-95.
As always, Weart’s historical review is worth re-reading: http://www.aip.org/history/climate/Radmath.htm
Fritz Moller may have come up with the first “modern” estimate for CO2 sensitivity — he calculated a CS of 1.5ºC for just CO2, no feedbacks:
Möller, Fritz (1963). “On the Influence of Changes in the CO2 Concentration in Air on the Radiation Balance of the Earth’s Surface and on the Climate.” J. Geophysical Research 68: 3877-86.
Moller’s model apparently fell apart when he tried to solve for H2O feedback.
The next significant advance (per Wearts) was Manabe, Syukuro, and Richard T. Wetherald (1967). “Thermal Equilibrium of the Atmosphere with a Given Distribution of Relative Humidity.” J. Atmospheric Sciences 24: 241-59. — they calculated 2ºC CS/2xCO2 with H2O feedback, a number that doesn’t look bad 30 years on.
HTH, PT
#6,7/ It would be far more helpful if you reported what these folks said that was relevant and how they tie in to the matters at hand.
#4 SM, I have seen arguments that local forcings inducing stable changes in temperature profiles are not physically possible from the simple equations, based on the POV that the energy throughout the profile must be equilibrated. The temperature profile in the upper atmosphere is another issue, as the density is so thin, the notion of ‘temperature’ breaks down somewhat, and its better to talk about pressure. I will try to find a paper on this tonight.
Bugs, are you seriously suggesting the technical reports of AR4 are for public consumption? The SPM, yes, the technical reports, no. Does these distractions ever cease?
The arguments that say the radiation depends on the temperature imply that the atmosphere also radiates as a black body. Likely a good assumption, except that we are specifically dealing with molecular exceptions to black body radiation. CO2 will radiate the very same frequencies it absorbs. If the energy absorbed by CO2 remains stored in vibrational and rotational states of CO2, then that is what it will be radiated as. The reradiated light will go up, down or sideways, and follow a random walk from molecule to molecule and eventually either be reabsorbed at the surface or emitted to space. The energy reabsorbed at the surface should keep it warmer. It can be reradiated anywhere in the black body spectrum from the surface, most of which will obviously escape to earth. If there is some mechanism that converts that stored energy into kinetic energy, it will be removed from that random walk and increase the local atmospheric temperature. Such mechanisms could be collisions, chemical reactions, or interactions with free electrons. Most of what I have read claims the latter two are rare, and I have yet to see any argument for the CO2 to cause local warming, but I am not an atmospheric chemist or atomic physicist. And I have yet to digest the very good looking references that have been posted at this site that make similar claims about the effect being dominated by the temperature where the radiation is emitted to space.
There are many possible physical effects that can be taking place and it is important to verify what is really happening. Something I haven’t seen mentioned is that N2 has vibrational excitation levels that are very close to some of the vibrational excitation levels of CO2 and is used in CO2 lasers to help populate the excitations. Since there is so much N2 in the atmosphere, this is a huge resevoir for storing the excitation energy absorbed by CO2 and a possible alternate route to transfering the energy to thermal energy. He is also used in those lasers to help cool the rotational excitations, but there is not much He in the atmosphere so that would be an unlikely occurance.
I am very curious and would like to know what they claim is really happening and how they have addressed all the sundry possabilities. And I am very disappointed at all the official arm waiving.
Convection forces the atmosphere to cool at a certain rate with height … about 6 K/km on average (the so-called lapse rate). Thus, convection leads to the mid-troposphere being several 10s of degrees colder than the surface.
You’re right, however, that convection is a net heat source for the mid-troposphere. Without convection, the mid-troposphere would be considerably colder, but that’s not the comparison Houghton is making.
“Now the proportion of radiation-to-space from the stratosphere in practical terms appears to be very small”
It would seem so; the stratosphere is 1/5 the amount of atmosphere as the troposphere, ranges
from starting 5 to 30 miles above the Earth, gets up to only about -3C at the top (from around -50C at the bottom), has plenty of ozone, and the chemical reactions of methane (brought by rising air in the tropics) with hydroxyl radicals is the main source of water vapor in the stratosphere.
How much of each GHG and AGHG is in the stratosphere compared to the tropopause and below?
This is a great web site and I am very much a lurker. I have a degree in science (BSc Chemistry) so I am capable of understanding about 50% of what is said on this site.
However, a true test of the CO2 GHG gas hypothesis (I will not grace it with the title Theory for now) would be its ability to make predictions and not to fail a crucial test. So my question would be:
What 10 (you can add more) predictions does the hypothesis make? Does it fail any of them?
Is there a crucial test that could be made that the hypothesis fails?
Oh, what we don’t know; remember 2006?
Plants release methane with or without oxygen. Who’da thunk it.
Minor complications which you probably already know about – the tropopause varies from 8km at the poles to 17km at the equator, and of course the temperature at ground level varies over a wide range too. The arithmetic means of heights and temperatures are not necessarily the appropriate averages to use. The lapse rate in dry air is a consequence of vertical mixing (caused largely by convection – hence Houghton’s comment) causing it to expand/contract without exchanging energy with its surroundings. The expansion with altitude cools it, the contraction with descent warms it. Convection transports heat upwards, but in so doing the temperature of the air carrying it drops. Since much of the greenhouse amplification is said to be due to water vapour, which mostly exists mainly in the lowest few of km of the warm troposphere, the height from which it radiates into space will be lower.
Sorry that’s talking about physics rather than the IPCC references you wanted. It might be nice if the IPCC explanations mentioned some of this stuff.
Looking at the spectra graph – CO2 is shown with the 667 cm^-1, but CO2 also has has a second band off the graph at 2349cm^-1.
Water vapor should have 3 bands 1595, 3756 and 3652 cm^-1 – is the minimum at the right the center of the first one of the water vapor bands (spread very wide)?
The narrow peak is probably due to emissions from CO2 that is emitting and under so little pressure that it isn’t interacting with other molecules. Is the spread in frequency due to the vibration-rotation-bands?
Interaction between molecules should also produce ‘overtone bands’ (combination bands of sums and differences) – perhaps they are too small a magnitude to be seen?
It would be nice to see a similar graph that covered a few more octaves and a comparison of land and ocean areas.
Steve Mc: Isn’t the diagram you are looking for similar to Figure 1 (p. 447) in Held and Sodden 2000? The “higher-colder” concept just refers to the lapse rate.
This problem is way more complex than is being described here. To start, discussion CO2 is ignoring the 800 lb gorilla in the room, water vapor. While the halfway point for CO2 may be 5 or 6 km, the halfway point for water vapor is of order 2-3 km. And since water vapor thermal effects far outweigh those of CO2, it is going to dominate.
Much also depends upon the relative temperatures of the earth and atmosphere. I can’t figure out how to embed a figure here. But figures like #3 in the text by Steve isn’t universal. With different temperature relationships, it may look inverted, i.e. the places where you see absorptions in figure 3, there will be emissions.
Lastly, be careful not to overestimate the effect of the high temperatures high up in the atmosphere. The density of the atmosphere at just 60 km is of order 1/10,000th of that near the surface. The amount of radiation emitted by a km of atmosphere is proportional to its density. The thermosphere may be hot, but its density is a millionth that of the surface.
It seems that we’re discussing the explanation of the GHE and EGHE in at least three different threads. I understand that Steve McIntyre started each of them out as a critique of the explanation provided by different sources: two issues of the IPPC report and now this textbook by Houghton. However, it seems that there is a lot of going over the same ground several times amongst these threads, at least wrt the basic structure of the argument.
I certainly agree that there is a hole in the literature for a self-contained consistent end-to-end explanation of the whole argument. What I can see from trying to uphold one end of the discussion is that it becomes difficult to keep the discussion self-contained, because you have to drag in more and more radiative-transfer theory, some basic quantum mechanics, information about blackbody radiation, and perhaps a few other topics.
One of the more helpful things I’ve seen is Weart’s book/website, which presents the history of the global warming discussion. What one sees there is that there were several generations of students of this problem (it’s been studied over 100 years), and the problems and misunderstandings that the pioneers had are still being stumbled over by new entrants to the discussion today – even though they have actually been resolved.
I admit to being particularly disappointed in the explanation provided in Houghton’s textbook on GW (actually I think there are two: one very simplistic (the one Amman provided before) and the other a more complicated but not really more accurate model), because Houghton’s book on the physics of planetary atmospheres provides, in brief form, all the basic tools to construct a real explanation. But even there, he scatters the derivation over the entire book, with crucial steps left as exercises.
There is still a space in the literature for a nice clean exposition of the standard theory.
I don’t know if we’ve yet established that there is a single standard theory. I think that’s what we’re trying to nail down.
Neal (#20), IIUC, the underlying atmospheric physics — i.e. classical physics plus a smattering of quantum — are well understood in the sense that we can model the “behavior” of a “finite element” of atmosphere given its internal properties and appropriate boundary conditions. So that part of the problem is solved, right?
The follow-on question is whether we can derive — through clever approximations and elegant numerical tricks — a useful analytical expression for large-scale behavior based on this micro-level understanding. In theory, I suppose, one could apply brute-force numerical methods and get an answer for a specific case — but would that address SteveM’s question?
It seems that many complex systems (biological ones come to mind) have this annoying property: It is hard to connect what we understand about the micro structure to the behavior we observe at the macro level.
Am I missing the point here?
Larry–
Actually, I’m groking what Neil is saying.
On the one hand, there really is no standard theory. But, on the other hand, there are between 3 and 10 sort of standard approximation. There is the O-D model. There is the 1-d isothermal earth at steady state model with various levels of details for the atmosphere. I’ve seen 2-d approximations. Etc.
I’m thinking some work does need to be done to put up whatever it is the climate scientists this is ‘too much’ for the general public, but is still required for people like you, me, Neil, Pat, etc. We all want more detail than those IPCC color cartoons with no math or physics. But, we don’t really want “Run a GCM” as an answer either.
Each of us has read bits and pieces of different formulations and we are all arguing without doing what engineers and scientists usually do– draw things at a black board, write down our assumptions, write equations and show the solutions.
#22, TAC:
I don’t see any reason to insist on an analytic expression for the end result. No less a theorist than Richard Feynman told me not to get too hung up over the issue of representing results as columns of numbers vs. as a series of Bessel functions. I think what is important is that we should be able to agree that according to the generally accepted principles of atmospheric physics, the result of a 2X in C-O2 content really is 3.7 W/m^2 (or whatever); and that this results in a specific new steady-state GAT, if one doesn’t worry about feedback loops.
In careful contradiction to lucia (#23), I would say there is a standard theory (including radiative transfer, no Stefan-Boltzmann, focus on a few main IR bands); but there is not a standard calculation (could be 1-d or 3-d, etc.). We don’t need a GCM to do this calculation, but we do need a better explanation of what has been done.
On the other hand, to calculate the temperature sensitivity with feedback loops, we definitely need a GCM. We know that’s got to be complicated; and we know that we don’t yet know how to take everything there into account.
@Neil– 24. NP. I supposed the distinction between theory and method of calculation relates to all the different ways of obtaining numerical values by inserting various approximations? Yes.
I agree we need a better explanation, and some numbers sprouting out of it. I also have not need for an analytical solution, but I do need an analytical framework. The colorful cartoons SteveM has been posting (from IPCC) are not the useful thing.
Isn’t the “funnel” in the spectral analysis image also partially or mostly attributable to H2O, Not just CO2?
Now when CO2 or H2O absorbs radiation I would think that some or most of that energy is transfered to its surroundings, N2 and O2. Those gases would then radiate a portion of their energy in their own spectral pattern.
Doesn’t thermodynamics say that without work heat always flows from hot to cold. Therefore CO2 or any portion of the atmosphere should never radiate downwards in the troposphere because of the lapse rate. Of course as the troposphere increases in temperature from absorbing radiation it lowers the temperature delta thereby reducing the flow of heat from the surface to the tropopause.
Radiation neat but what about convection? Is it even possible to determine the relative importance of radiation vs. convection heat transfer in the troposphere to the altitude where LW radiation predominately escapes to space?
-chico
#12 Bill
It is not clear that convection is responsible for the lapse rate at all.
It can be derived as g/c where g is gravitation accelleration and
c is specific heat of the gas. Moreover, there must be some
temperature difference between the radiation warmed surface of the earth
anddeep space. I am not clear how even the current profile
in the figure can be predicted from physics, let alone
enhanced GW.
As far as the physics underlying the greenhouse effect goes, I think the steel greenhouse explains it the best. Here’s how to build one.
First, find a planet in outer space with no nearby star and no atmosphere, and heated solely by internal radioactive decay to a temperature of say minus 19°C (237 w/m2). The planet loses energy, of course, purely by radiation to space.
Now just build a thin steel shell that completely surrounds that planet. Make the shell say ten kilometres above the surface everywhere, with no connection to the planetary surface.
What will be the eventual equilibrium temperature of the planet and the steel shell?
Well, the planetary radiation will continue to heat up the steel shell until the shell is radiating outward at the same rate as the planet, 237 w/m2. At that point, the shell is radiating outward every single watt/m2 of the energy it is receiving from the planet inside, so it is in stable equilibrium. The temperature of the shell at equilibrium, of course, would be the same as the starting temperature of the planet, -19°C (237 w/m2).
But what about the temperature of the planet? To calculate the planetary temperature, we need to remember that the steel shell has an inside as well as an outside, so the shell must also be radiating 237 w/m2 inwards, towards the planet.
The planet, then, is receiving 237 w/m2 from the radioactivity, and 237 w/m2 from the steel shell. Thus, the planets new equilibrium temperature is 29°C (474 w/m2).
It is a curious and little known fact that the reason the greenhouse effect works is simply because a shell has 2 sides, an outside and an inside. Inherently, the greenhouse effect has nothing to do with CO2, or atmospheric concentrations, or greenhouses, for that matter. You dont need glass or an atmosphere, steel works just fine.
That’s my explanation of the underlying physics of how the “greenhouse effect” heats the surface of a planet. Will you find that explanation in a peer reviewed article some where? I doubt it.
Regarding the “enhanced greenhouse effect”, it refers (as I understand it) to a standard greenhouse with a (vaguely described) net positive feedback. This idea of net positive feedback seems like nonsense to me, parasitic loss will see to that.
w.
I think there is a typo: instead of 29 C (474 w/m2) should be 9 C (474 w/m2).
Neal (#24), Lucia (#25): Thanks for the responses. While I accept your judgment that there is no need for an analytical solution, it sure would be nice to have one. I, for one, am not entirely satisfied by the alternative of a finite-element numerical “solution” whose validity may depend on myriad, possibly unstated, computational details. Such “solutions” raise concerns both about the correspondence between the “model” and “reality” — we always have to worry about that — and also about whether the computational approach is satisfactory.
In particular, it is worrisome to learn that current GCMs are constrained by computational resources and employ (IIUC) finite elements representing 100s to thousands of cubic kilometers of atmosphere. Makes me wonder about the sensitivity of computed EGHG effects to computational details. Are such sensitivities being carefully evaluated? I have not studied the GCMS, and maybe everything is OK. Still,…
Along a different line, can the EGHG effect be obtained directly from observational data? Over what time frame (i.e. range of CO2 concentrations) do we have data for atmospheric chemistry and temperature profiles?
#28 Thanks Willis. The thing is, could this steel planet idea be used to predict the actual temperature profile of earth, from first principles? Do you know of any models or paper that attempt to reconstruct the measured profile, up to the thermosphere, using g, R and the composition of gasses?
#28
But Willis, the steel shell – for each square meter, it receives 237 Joules of energy each second from the planet, and it is radiating 237 J out to space. How can it also radiate 237 J inwards during the same second? Where do the extra 237 J come from?
Or am I missing something really obvious here….
Willis #28
The surface area of your shell is larger than the surface area of the planet below, so the watts per square metre of the shell must be less than that of the planet. As a geoscientist I dispair at the continual use of 1D models to describe the physical properties of 3D planets.
When figuring the chances of a re-radiated photon being captured vs. escaping to space, do any of the models take into count the curvature of the earth?
At it’s simplest. Do they assume that a photon emitted horizontal to the ground, will stay in the atmosphere until it is reabsorbed?
A little more complicatedly, with the exception of straight up, because of the curvature of the earth, the edge of the atmosphere is closer than a non-curved model would suggest. The further you get from vertical, the greater this error becomes.
Additionally, this discrepancy will also increase the higher up you go. At the surface of the earth, your horizon covers half of your field of view. (Assumping a perfectly spherical earth) As you get higher, the horizon shrinks. In other words, at the surface, a photon that is re-emitted at an angle of less than perpendicular to the earth, will hit the earth. It can’t escape to space. Go up a mile. Now a photon that is emitted at an angle of less than perpendicular to the earth, will miss the earth, and if it avoids re-absorbtion, can escape to space. (Admittedly, near the ground, the chances of that happening are slim, but they aren’t zero.) As you go higher, this affect increases. A flat model will assume that any photon emitted at less than perpendicular, will always be either absorbed by the atmosphere, or by the earth. This is incorrect, and it increases the apparent influence of any GHG.
Willis, since the steel shell is above the planet, it will have a greater surface area than the planet. As a result it will have to radiate less energy per square meter than the planet did in order to reach equilibrium.
Other than that, the difference in temperature of the planet’s surface will depend on the resistance to heat flow of the steel.
#1
Can something be found in the graphs in the .pdf at the following link?
Mark #34
You are quite right, it’s a matter of solid angles. There is always more vector “up” than “down” in the atmosphere, even at the surface. The tangent plane to a spherical surface only touches that surface at one point.
“Willis Eschenbach” why bother with a steel shell? Do the same thought experiment with the planets crust, the same logic indicates that just under the crust is twice as hot as the surface.
Sill, it is a good way to heat a house, just surround it with a steel shell and wait for it to warm up oithout energy input; though of taking out a patent?
NJK #24
Long time lurker very infrequent poster. Background is financial maths, with chemistry and physics thrown in where acceptable to degree requirements. You state that we need GCM’s, I agree with one caveat: We need GCM’s that tell us something. In the financial world, we model all the time. We do it in the standard way, observe, postulate, test it against historical data, and then come back and test it for predictiveness. If it fails this test, it is examined to make sure the parameters were faithfully applied etc. checked for bad code, etc. and redone. If it fails this, the hypothesis is scrapped. We don’t peek at the new data and do “ad hoc tuning”, because that will vaporize real money. Take LTCM. Their math was correct, but with only one faulty assumption, billions were lost. Any model with dozens of assumptions, the interactions between which are not even understood is useless, or worse dangerous, if future actions are taken based upon it.
The GCM’s can’t even get the sign right in many cases, they are worthless. The fact that the IPCC allows them to look at the data each year, and then go back and make unscientific adjustments to force the output to conform to observational data tells any objective observer everything they need to know about the provenance of GCM’s.
If the estimates for (if I remember correctly) 3.7 W/m2 for an doubling of CO2 were calculated using a flat model, then that number is too high.
How much? I don’t know. Probably not a large amount.
PeterT:
Worse still, they go back and make adjustments to the data so that it better fits the models “predictions”.
Two points Willis,
1. There does need to be a slight adjustment of the temperature of the shell due to the fact that the radius of the shell and thus the area of the shell is greater than that of the surface.
2. The earth’s situation differs from that of your steel shell in that some radation does go directly to space, sort of like a shell with some holes in it. Thus, the Enhanced Greenhouse effect corresponds to covering some of these holes and thus increasing the surface temperature.
Willis #28:
Interesting. Is this related in any way to Gauss’s Law?
First of all, thanks Steve for this thread, which addresses a very important area.
As far as the tropopause is concerned, the curve shown at http://en.wikipedia.org/wiki/Image:Atmosphere_model.png
is from a Naval Research Lab model of the atmosphere.
This suggests that the tropopause is an extended region — temperature really doesn’t rise significantly until about 20km.
I have seen similar spectral radiance graphs (fig 3) in other sources and it is attributed to one of the early Nimbus satellites. Why are the peaks of the black body curves different from what Wien’s Displacement Law gives? I realize that Wien’s law has a different constant for photon/sec (fig 3) or watts.
In regard to the lapse rate comment by Houghton, I think that the issue is this:
There is an adiabatic lapse rate, which is calculated on the basis that kinetic energy (and thus temperture) of a parcel of atmosphere is lost to provide gravitational energy (the mgh bit). There is a more-realistic lapse-rate, the environmental LR, which is smaller because there IS warming of the upper air layers, and the adiabatic assumption is thereby violated. This warming is due to both convection and radiative absorption.
#28
I get slightly confused…
Never mind the fact that the shell has a larger radius, and thus a different energy flux _density_. What really confuses me is that you in one paragraph state that all the energy is radiating outwards, while later you state that it also radiates inwards. Both statements can’t be true.
@agn–
That’s the way radiation works. Heat is radiated in all directions. So, if you have a plate, it radiates from both sides of the plate. A shell is sort of like a big plate, but curved to close in on itself.
@Stippen–
The issue of all radiating outward in willis part 1 has to do with how one approaches the problem.
If we had a blackboard, and willis were lecturing students, he’d probably draw a control volume for the first argument. That volume encloses both the planet and the shell. He applies the conservation of energy on what gets into and out of the volume. He gets a solution for the temperature of the shell. The radiation from the inner surface of the shell doesn’t matter in this part of the problem.
Then, he would move an and draw a second control volume. This one is around the planet, but the shell is outside. Now, he applies conservation of energy again and solves for the temperature of the planet.
I think you can see that if you haven’t done this problem before and you don’t automatically “see” the two control volumes as you read Willis’s post, you get lost when he jumps from the first illustration (not shown at the blog) to the second illustration (also not show!). If you’ve drawn control volumes over and over, willis’s argument is easy to follow. Otherwise, it’s impossible.
(Note to all: Peer reviewed articles do already solved problems, willis’ way and then cite a reference. You assume the audience is sufficiently familiar with certain conventions that you don’t take 3 pages to show them. The engineering exposition is the way I say you show students who have sufficient background to follow what you are doing, but who are in the process of learning certain conventions. That’s kind of the difference. Peer reviewed articles in engineering do exactly waht peer reviewed articles in science do. The key word in “engineering exposition” is “exposition”, not “engineering”. Scientists get involved in engineering expositions too.)
@Philip @Dave–
Philp, don’t despair too much about 1-D models. I’m sure willis means a very large planet, and a very small gap between the planet and the shell. So, the area ratio are: (1+δ/R)**2. Say the planets radius is 6,500 km and the shell is set 60 km out. Then δ/R ~ 10^-3 or something like that. You can adjust Willis calculation, and then round to 2 significant figures. Now, adjust for the area, and you’ll find the difference is buried in the roundoff. (Dave– This is how slight the correction is likely to be.)
@Mark–
No. Ultimately, photons can escape. Even in a very thick atmophere, if looked at from the level of photons, there is always a possibility that an individual photon emitted from the surface of a planet can escape.
There are a variety of ways to formulate the problem to do a calculation. In a contiuum approach, you don’t even think about photons, just as when someone writes the Navier Stokes, they don’t think of individual molecules. But, there are always underlying physical arguments that explain why the Navier-Stokes is appropriate at a continuum level, and similar things happen for radiation. With a radiation problem involving a participating media (the atmosphere), the underlying problem for a continuum formulation assumes individual photons can, ultimately, escape an atmosphere of finite depth.
When you reach the upper atmosphere, you do need to think about photons. I have no familarity with what successful approaches are used there, but it appear that Phil, Neil and Larry do. But, I’m guessing that, as in all problems, the issue of the curvature of the earth can sometimes be neglected and other times not.
The answer to your question will be similar to that for Philips above– if sphere is large enough, then, for all practical purposes, locally, curvature can be negleted. When we get to a particular problem, that can be discussed.
@DocMartin–38
Willis’s argument requires an airless gap separate two solid surfaces. The gap prevents conduction and radiation. His problem is related to the greenhouse effect, but, alas, not quite. The greenhouse effect is a participating media problem. It does not involve a solid surface outside.
However, the shell is useful as a heuristic device. That shell represents a resistance to radiation transport, in that way, it describes what the effective behavior of the gas. In fact, I think the simplest explanations of layers of gases with optical depth of “τ=1” gives numerical results that match willis’s example, if you assume the heat is actually created in the core of the earth.
Segue to @Dave #42 point (2) Relating what I said to Doc to your point 2: The underlying derivations to come up with optical depth permit individual photons to escape the layer, but you stop thinking about photons when you switch to the continuum point of view. He’s moved the source of heat to the core of the planet. He’s mimicke atmosphere with an optical depth of τ=1 with a solid shell. (The shell problem can only have interger optical depths. Real atmospheres can have fractional optical depths.)
So, Willis’ argument is closely related to the real radiative convective argument before you add the convective correction, but no, it’s not the same problem.
==========
It is pretty obvious all these types of questions do need to be addressed somewhere. They are asked over and over by people who know some physics, heat transfer, thermo, fluid mechancis, but don’t want to spend 40 hours a week for a month doing a literature search to get answers to and endless series of questions that occur to them. The questions include things like, “what about the curvature of the earth?” These people also don’t want to just have someone say “Look. I have a Ph.D. I’ve studied the problem. I’ve thought about my assumptions a long time, and I’ve decided they are good. Trust me.”
Many of these questions have answers, but they take time to address. The discussion often require figures and equations.
For some reason the IPCC hasn’t created these documents, nor have the gov’t. agencies. Peer reviewed articles are great; text books are great. But neither of these takes the place of the full document that is required: an full engineering exposition.
I think Neil is correct that the full exposition could be condensed down to a peer reviewed article, with details like showing that it’s really ok to neglect the curvature of the earth for certain purposes (but not others). Strangely, blog discussions are suited to answer the answerable questions. It’s just that the proper blog doesn’t currently exist.
Re #10 dover_beach says:
January 8th, 2008 at 4:05 pm
Bugs, are you seriously suggesting the technical reports of AR4 are for public consumption? The SPM, yes, the technical reports, no. Does these distractions ever cease?
Bugs is correct. The “technical reports” do not read, look or allow extrapolated calculations as a technical report would. Public consumption as target audience is obvious from the lack of mathematical formula. In publishing, there is a need to set up front the style and depth of mathematics permitted in the text as it has been said “for every equation the readership goes down by a factor of ten”.
#28 Willis Eschenbach:
What happens when you build a second steel shell outside of the first?
Look. I have a Ph.D. Ive studied the problem. Ive thought about my assumptions a long time, and Ive decided they are good. Trust me.
Lucia,
It’s kind of a strange situation where we’re supposed have have a consensus on the end result, but no consensus on how to get there.
Pat, it is a whole lot more complicated than that, which is why I’ve been trying to discourage all of this over reliance upon the super simplified models. Not only is the tropopause thickness and height quite variable, it also FOLDS deep into the troposphere between supercells along their fronts. The complex and varying geometries involved along with the interactions with the jet streams makes all of this speculation about the models of adiabatic rates exceedingly dubious and unlikely. You can gain at least a beginning appreciation for the complexities involved by simply looking at yet another simplified diagram and profile of the atmosphere:
B. Geerts and E. Linacre. The height of the tropopause, 11/’97
The super simplified models are useful for conveying some general ideas about the tropopause, but they are also exceedingly unuseful when they are mistakenly taken too literally and neglect major features and effects of the tropopause.
Re#28, Willis Eschenbach:
Your steel shell around the planet should have 3 surfaces, one facing outward and two facing the planet, to satisfy IPCC GHE theory requirements. According to canonic IPCC illustration:
Atmosphere GHG radiate into space 165 W/m^2, while returning to Earth 324 W/m^2 as back radiation.
lucia:
That’s not the question that I asked.
If a photon is headed toward the earth, it can’t escape. If the photon is headed parallel to the earth in a model where the earth is assumed to be flat not curved, it can’t escape.
Lucia, how does your argument change when you replace your solid with a fluid?
Air is an excellent insulator, as long as no convection is present. Allow convection, and it’s pretty efficient in transferring heat.
Sorry wrong attribution. I meant to address Willis Eschenbach #28.
54 D Patterson
I don’t disagree with the complexity you talk about, but the thunderstorm folding has to be considered as an atypical, localized situation, not a typical one.
The problem I have with the link you gave is that the authors don’t define what they mean by ‘tropopause’, and discuss it as if it were a thin well-defined surface, which it is not.
@MarkW — 56
That makes no sense Willis. Are you trying to do a thought experiment to show the models are inadaquate? 🙂
We would need to know more information to figure out what’s going on with that shell and that planet. The planet is producing 237 w/m2. Fine.
What’s the surface of the planet made of? Once we put the shell around it, we would need to know if the surface would react to heating up the space between the planet and the shell to some temperature. Is it dust? Iron? Methane? Ceramic tile? Jello?
We would have to calculate the volume of the space between the surface and the shell; what is the heat transfer rate of vaccuum, +/- any reaction of the surface of the planet, +/- and how much heat the surface can absorb (or not) over whatever it was doing without the shell.
We would have to calculate the surface area of the shell, so we’d need to know the size of the planet to find out how big a shell 10 KM above the surface is.
We would have to know the thickness of the shell, as it takes energy to heat up the until now temperature of space X amount of steel on the inside (internal surface area), versus X+Y on the outside which is affected by the temperature of space (internal surface area+thickness of the steel).
I would invision that the 237 w/m2 (assuming a non-reactive planetary surface that can not hold any heat on its own) would be used somewhat in heating the shell on the inside, which would most, if not all, (depending on thickness) be transferred out into the non-heated space on the outside.
How much re-radiation back to the planet would you get with metal heated by 237 w/m2 coming from inside and space coming from outside (2.725 K; assuming you’re not setting up the situation where there is no background microwave radiation) What type of steel? What’s the heat transfer rate through type times thickness? Don’t forget, the interior now no longer has that 2.725 K reaching it, that needs to be factored in also.
We’d probably also need to know a bunch of other stuff about the planet also (shape, rotation, orbit, tilt of axis, and so on) unless you’re setting up the situation so it’s just a planet sitting there in space.
I do not believe in the Enhanced GHG effect. I do not think that extra carbon dioxide will have any effect on the Earths temperature.
Firstly, I should like to refer Spencer Wearts article on the Real Climate site entitled A Saturated Gassy Argument, SGA.
http://www.realclimate.org/index.php/archives/2007/06/a-saturated-gassy-argument/#more-455
This work implicitly seems to accept that carbon dioxide and water vapour can absorb 100% of the infrared radiation from the surface of the Earth. Most of this energy is radiated back to the surface. See Kiehl and Trenberth
http://www.cgd.ucar.edu/cas/abstracts/files/kevin1997_1.html
This is the natural GHG effect, and occurs at relatively low altitudes. No argument. For the purpose of easy discussion, let us consider the atmosphere to consist of three regions, lower and upper troposphere, and “high altitude”, as in SGA.
The infrared which is not radiated back to the surface heats the troposphere by increasing the kinetic energy of the molecules by inter-molecular collisions.
Heat energy also leaves the surface as sensible heat, convection and latent heat, which rises to the upper troposphere where it too provides warming. Kiehl and Trenberth show that sufficient energy is provided to this region by these means for the necessary 165 Wm^-2 to escape to space in order to enable energy balance for the Earths system. ( Together with 30 Wm^-2 from the clouds and 40 Wm^-2 through the window. ) I suggest that the available energy is converted into the necessary photon form by inter-molecular collisions with CO2 in the upper troposphere and at high altitudes.
Some of the photons about to escape into space at high altitudes will be absorbed by CO2 molecules even at this height, as stated in the SGA, but it is at this point that my ideas diverge from the SGA.
The excited CO2 molecules will decay again to a lower rotational level, either spontaneously or by collision, so emitting photons. Some of these emitted photons will escape to space as required, and some will return to the atmosphere, but the temperature will be adjusted by the overall feedback system so that energy balance will be maintained.
It is important to understand that only a small proportion of the total atmospheric CO2 is involved in this process. The relevant actions are occurring at high altitudes where the air is very thin, after all, not throughout the whole atmosphere. This proportion is dependant on the temperature of that region. The number of CO2 molecules involved will be set at this temperature to provide the required number of outgoing photons to get energy balance.
If now, more CO2 is added, more photons will join in the process and escape to space, so tending to reduce the temperature, and upsetting the balance. Correction is provided by the emitting region moving to still higher altitudes, ( in line with the SGA ), where the temperature is lower, and so the emission rate is reduced, as required.
Note that the number of CO2 molecules involved must be constant, so that when more CO2 is added, the proportion involved is reduced accordingly.
I suggest that final escape to space occurs at higher and colder altitudes because the proportion of CO2 molecules participating must be less, not greater, when more CO2 is added, in order for equilibrium. The total number of CO2 molecules participating has to stay constant.
The problem with the SGA is that it stops too soon for the flaw to be seen. The crux of the SGA is where it refers to extra CO2 molecules absorbing photons at high altitudes. It neglects to say anything about the next, inevitable process, which is the following decay, with the centralised energy packet within the excited molecule distributing its energy into the various states available to it, in line with the Second Law of Thermodynamics. Must not forget that!
There is a graph of the “absorption spectra for major greenhouse gases in the Earth’s atmosphere” at the following address: http://www.iitap.iastate.edu/gccourse/forcing/images/image7.gif
The lower curve is for the total atmosphere. Water vapor is quite obviously the major factor in the overall spectrum.
#20, Neal
Yes, it’s confusing. Steve, you may want to combine the threads, if that’s practical, or perhaps a knowledgeable, energetic reader (whose initials are NJK? {GG}) could post a summary with links.
And I certainly agree re Wearts — see #7 above 😉
#24,
Now, there’s an impressive name-drop!
Cheers — Pete
@lucia #49
Thats the way radiation works. Heat is radiated in all directions. So, if you have a plate, it radiates from both sides of the plate. A shell is sort of like a big plate, but curved to close in on itself.
Yes, lucia, I think I understand that. But since the shell is for all practical purposes the same size on the inside as on the outside, it would appear to be radiating twice as many units of energy (Joules) as it is receiving. And I thought the first law of thermodynamics had a problem with that sort of thing.
Re: The Willis Eschenbach Steel Greenhouse (patent pending)
INSTRUCTIONS FOR BUILDING A STEEL GREENHOUSE
Again, why would a metal surface with heat on one side and basicially zero degrees on the other radiate anything back? At the least, you’d have to work out the transfer of the energy to the steel (and what heating that creates with a lack of atmosphere and the loss of the background microwave radiation on the inside) and then the reaction of the steel to the energy, and then the effects of the 2.725 K on the outside.
What temperature does space reach with no background microwave radiation when its encased between a planet surface emitting 237 w/m2 and a steel casing? What effect does the outer 2.725 K have upon the inner X K?
agn #65 — What you are missing is that the planet will heat up until it emits 2×237=474 W/m2 at equilibrium. The planet as one “control volume” is receiving 237W/m2 from radioactive decay and 237W/m2 back from the shell. It is emitting 474W/m2 (once it has heated up) of thermal radiation, so it is in equilibrium at this point. The shell as a separate control volume is receiving the 474W/m2 from the planet, and emitting 237W/m2 back down, plus 237W/m2 “up” out to space, so it is also in equilibrium. The planet/shell combination is receiving 237W/m2 from the decay, and emitting 237W/m2 to space, so it all checks out. As lucia says, if you’ve ever taken a thermo class, you are familiar with this type of analysis; if you haven’t, it takes a while to get used to. To me, this is a classic Thermo 101 example.
I have recently been having a problem with accepting Real Climates Saturated Gassy Argument. As far as I can see, it is incomplete and so it is also misleading. Please refer also to my #62 above.
Please consider the following.
Let C = total number of carbon dioxide molecules in the pre-industrial atmosphere at 280ppmv
k = the increase factor in CO2 concentration relative to pre-industrial conc. of 280ppmv.
s = proportion of emitted photons escaping to space
win = total number of photons escaping to space through the window per unit time
For CO2 increase factor k, let
b = proportion of carbon dioxide molecules excited by absorption of photons, and
intermolecular collisions
Then, number of CO2 molecules excited by absorption/collision = kbC
All these molecules emit photons.
Let the following expressions apply for unit time, where p is the appropriate constant of proportionality.
Then in general, we have:
Number of photons escaping to space = pskbC + win .. (Eqn 1)
Now consider the case of the pre-industrial atmosphere.
We can put k = 1 and b = b1.
Then, number of photons escaping to space = psb1.C + win …..(Eqn 2)
Now in energy balance conditions, the number of photons escaping to space must be constant.
Therefore, from Eqn (1) and Eqn (2), we have pskbC + win = psb1.C + win
Hence, kb = b1
But b1 is a constant.
So as k increases, b must decrease for this relationship to be satisfied and energy balance to be maintained. That is, as the amount of carbon dioxide is increased, the proportion of the number of CO2 molecules participating in the process is reduced. This requirement can be accommodated by a fall in temperature from the pre-industrial value at high altitudes.
This means that increased CO2 produces extra COOLING at high altitudes.
What happens in the atmosphere?
In general,
Number of photons returning to the atmosphere = p(1 s )kbC …. (Eqn 3)
And for the case of the pre-industrial atmosphere, k = 1 and b = b1, as before.
So, the number of photons returning to the pre-industrial atmosphere = p(1 s )b1.C ..(Eqn 4)
Therefore, the change in photons returning to the atmosphere = p(1 s )kbC p(1 s )b1.C
= p(1 s )C(kb b1)
But, in energy equilibrium, kb = b1.
Therefore, the change in the number of photons returning to the atmosphere = 0
This means that there is no change in the temperature of the atmosphere due to increasing the amount of CO2 present.
My thanks to everyone for their comments. Philip M, you say:
While this is true, the difference in area is so small (less than half a percent) that it is usually ignored in the calculation.
AGN, you say:
Not so. Let’s call the heat received from the radioactivity, (237 w/m2 in the example above), “S”. At equilibrium, the planet receives S watts/m2 from the internal radiation, and it receives another S watts/m2 from the shell. The final temperature of the planet is 2S, and so that is the amount that it radiates. Not S, but 2 * S. Here’s a drawing of the situation, with and without the shell.
Sam U., you have pointed out that I have neglected the background radiation temperature of 2.725°K, which is true. As this 2.275°K equates to 0.000002w/m2, however, I think we are justified in this particular simplification. You also ask “Again, why would a metal surface with heat on one side and basicially zero degrees on the other radiate anything back?”. The radiation does not depend on what is around it. Hot metal radiates, regardless of what is around it on one side of the other.
Pochas, you raise a very good question when you say “What happens when you build a second steel shell outside of the first?”. The answer is that the equilibrium temperature becomes 3 * S, instead of 2 * S. The question is important because A SINGLE SHELL GREENHOUSE DOESN’T EXPLAIN THE EARTH’S TEMPERATURE. I put that all in caps because it is not widely known. Even our own beloved Judith Curry uses it as an example in her textbook, despite the fact that it cannot possibly be correct.
The reason it can’t be correct is that a greenhouse has a maximum temperature, which is dependent on the number of shells. The earth receives about 235 w/m2 from the sun. With a single shell greenhouse, the maximum possible temperature of the surface is ~ 470 w/m2. But we know that we have at least ~100 w/m2 in losses (convection/conduction and evapotranspiration), which only leaves 370 w/m2 (11°C) as the max possible surface temperature. This is well below the actual surface temperature of the planet, which means that we must have at least a double shell greenhouse system.
w.
Steel Greenhouses and Radiative Heat Transfer
See the Introduction in Chapter 1 and then Chapter 10 in this free online textbook. Section 10.4 has all kinds of examples including spheres and heat shields/steel greenhouses. I kind-of recall, and it’s been a very long time, that we used the energy conservation principle to setup equations so as to calculate the temperature distribution among the surfaces and these give the heat transfer and/or power. Iterative numerical methods are generally required because of all that fourth-power stuff. But maybe not for concentric spheres.
“Curt says: #65 The planet/shell combination is receiving 237W/m2 from the decay, and emitting 237W/m2 to space, so it all checks out. As lucia says, if youve ever taken a thermo class, you are familiar with this type of analysis; if you havent, it takes a while to get used to. To me, this is a classic Thermo 101 example.”
No it is an example of applying classical equlibrium thermodynamics to an essentially irreversible theromodynamics problem. The solution is wrong and I shall demonstrate why it is wrong and the true answer.
By you logic
on could take a small unranium mass and place it at the center of a series of spheres, each seperated by a vacuum. The system would heat up so that the total radiation output of each of the sphere surfaces would double as one ventured inward. Build enough spheres and the uranium is hotter that the surface of the sun.
What you forget is the system is no a equilibrium, it is at steady state. Taking the one spehere example, this is what is going to happen. The outer surface CAN only radiate outward, into deep space, every photon lost, is lost forever. The ouer shell is in true equilibrium with outer space and is at about 4 K. The rate at which the sphere is able to dump heat is far greater than the rate at which the underside is getting heated. All the energy absorbed on the inside will make its way to the surface and then rapidly bleed away. The outer sphere will be at the same temperature as deep space, always. The surface will experence no heatinf and it will appear at the planets surface that the steel barrier is not present.
Indeed, most space craft that use plutonium reactor powersources use black metal radiators to dump heat into space. The radiators need only be small., and they work and the radiators are near deep space temperatures (pu238 has a specific power of 560 watts per kilogram and the conversion is about 5%):-
There are only two things: Kinetics and thermodynamics. At equlibrium, processes can be simply described as kinetics cancel out. In non-equilibrium system, kinetics MAY decide the outcome. What you have described is a steady state, to calculate the temperature in a steady state you would need to workout the MAXIMIUM energy flux from the shells surface, this will be much more that suggested by the Stefan-Boltzman equation, as there the surface of the body is radiating both inwardly and outwardly at constant temperature.
You have described a wonderful example of why eqilibrium thermodynamics cannot be used to describe GHG in the atmosphere.
lucia #49
So the inverse square law does not apply to Willis’s model?
Philip 73 — Of course the the inverse square law holds. In the figure you show, at the link you cite, the first shell is 1 full radius away from the planet.
But willis did not put the spherical shell a full earth’s radius aways from the surface of the planet. He put it 10Km from the surface of the planet. Apply the same principle, but instead of setting the radius for the first shell at r=2, set it to r=1.001
Then, apply the inverse square law.
No. The shell is receives the heat radiated from the surface of the planet.
While it seems mysterious this is not the same as the heat from the nuclear reaction within, it’s not. It’s the heat radiated at the planet’s surface temperatures which is higher than the shell temperature.
But, when viewed by the cold universe, the energy coming off the shell has to be the energy from the nuclear reaction, because that’s the energy being dumped into the universe.
#70 Willis:
Willis, thank you for your reply. Actually, since essentially all 15 micron radiation is absorbed for the first time in the first 10 meters of atmosphere, there must be many, many “shells.” Yet we do not see the soaring temperatures this assemblage would generate. We see instead the adiabatic lapse rate, which is the falling temperatures you get from the reversible expansion of a rising column of convecting air. The modelers simply pick the number of “shells” that give the answer they want, and discard all the rest, without ever talking about convection, which is the true phenomenon of interest. But convection doesn’t yield Global Warming. You get at most some warming confined to the layer of air next to the surface, but little effect on the troposphere above, and I would guess little effect on absolute humidity. Any analysis of the type requested by Steve McIntyre would have to disclose this, so don’t hold your breath.
Why the hollow sphere is not a blackbody.
We make two polished iron spheres, both 50 cm diameter, but one is sold and the other is hollow and only 0.5 cm thick.
We take the two spheres and heat them in an oven (in space) to white hot, 1273K, and then remove them from the oven.
Both sphere are placed in the vacuum of space and monitored by a spectrophotomter. Both will radiate the same amount of energy at t=0, BUT, the hollow sphere will cool very more quickly. In the blackbody it is taken that the vast majority of heat transfer is by conduction. Take a space shuttle insulation tile from an oven and it glows, but you can lift it with your naked fingers, even though it is glowing.
Here is a film showing what happen when the rate at which a material can radiate heat is much greater than the rate at which it can transfer heat by conductance.
If the shuttle tiles were classical blackbody’s this could not happen. In black bodies the rate that heat is transported by conduction is alway much greater than by radiation. The sun is not a black body as it is much hotter inside than outside. The rate at which the interior can transfer heat is much less than the rate the outside can.
The hollow sphere is not a black body as a classical black body, a metal sphere, has the same heat transfer rate across its whole mass and all the mass of the blackbody MUST be isothermal. In a sold mass this is possible as heat conduction is faster that radiation can escape from the body. In a true blackbody only the atoms on the surface have as much chance to radiate inward and outward, all the others transfer heat outward by convection.
DocMartyn, you say:
Yes. If you take any heat source and insulate it well enough, it will get very, very hot. Not sure why you have a problem with that.
Hotter than the surface of the sun, however? Only if your metal shell can get hotter than the surface of the sun and not melt …
w.
PS – a final note. The radiation does not double with each additional shell, it increases by one. With one shell, the max is double, with two shells triple, and so on.
Re: Various
The interior of any closed object with reflectivity less than one (any real material) is a blackbody. In a closed container, any emitted photon will be eventually absorbed. It doesn’t matter how many times it’s reflected before absorption as long as the number of reflections is finite. You construct a blackbody for calibration purposes by making a small hole in a large container. It doesn’t hurt to paint the walls black, but it isn’t really necessary. The radiation emitted from that hole can be a very good (99.99+%) approximation of a theoretical blackbody at the same temperature.
A hollow sphere cools faster than a solid sphere because the total heat content is lower. Make the hollow sphere from a denser material than the solid sphere so the heat capacity is the same and they will cool at approximately the same rate provided that the spheres are small enough so the temperature gradient from the surface to the center is small (high thermal conductivity).
Wien’s Displacement Law is normally expressed in wavelength. The spectra above are in reciprocal centimeters (cm-1), which is effectively frequency. The Planck blackbody equation looks quite different when expressed in frequency compared to wavelength (See here).
The spike in the center of the carbon dioxide band is caused by emission from carbon dioxide in the upper stratosphere where it’s warmer than the tropopause and the optical depth is quite small. You can see the same effect in the ozone band at 1050 cm-1.
Radiation transfer calculations in the troposphere are done using the local thermal equilibrium assumption. That means that the probability of energy transfer from inelastic collisions with other gas molecules is much higher than the probability of photon emission. LTE holds to fairly low pressure. Because energy transfer to CO2 from collision with oxygen is very efficient, LTE holds for CO2 to about 100 km altitude. LTE means that the kinetic temperature of water vapor and CO2 are equal to the temperature of the other components of the atmosphere.
Nitrogen and oxygen can form collisional dimers that absorb IR in the thermal IR band, but this absorption is 5 or 6 orders of magnitude weaker than water vapor, so emission and absorption by these dimers is insignificant in the troposphere. Oxygen does have a magnetic dipole moment so it does have an emission spectrum at long wavelengths, but the strongest bands are in the microwave region where the energy density from thermal radiation from the surface and lower atmosphere is quite low. The 60 GHz oxygen emission band is what’s monitored by the satellite Microwave Sounding Units to measure atmospheric temperature.
Lucia #74
Radius of planet r (not specified) assume r = 10,000 km
Height of shell h (specified) h = 10 km
Thickness of shell t (not specified) assume t=0
Metric used in the calculation is Watts per square metre, parameter = 237 in posting 28
Wallis assumes surface area of Planet equals surface area of Shell, therefore for Wallis the Shell emits 237 watts/square metre.
Apply Geometric Correction because Surface Area of Shell > Surface Area of Planet.
Radius of Planet = 10,000 km
Surface Area of Planet = 1.25664E+15 sq m
Brilliance of Planet = 237 Watts per square metre
Power Output of Planet = 2.97823E+17 Watts
Radius of Shell =10,010 km
Surface Area of Shell = 1.25915E+15 sq m
Power Output of Shell= 2.97823E+17 Watts
Brilliance of Shell = 236.5267 Watts per square metre
Philip, you are correct. However, as I said before, the difference is so small (in your example, about 0.2%) that it is ignored for most calculations of the GH effect … our uncertainty in a host of other variables is much larger than two tenths of a percent.
w.
Thunderstorm folding? It appears as though you didn’t understand their descriptions and charts. Tropopause folding is certainly not associated with only microscale or mesoscale thunderstorm activity of a local or regional nature. The troposphere is not a single planetary scale atmospheric layer or shell. Generally speaking there are many tropopause layers, each usually being situated atop one of the supercells of the troposphere. Differing in altitude above adjacent circulation supercells, the latitudinal margins of the major tropopause layers overlap around the planet. It is in these marginal gaps circling the planet that jet streams form and the tropopause dips and frequently folds. Thunderstorms are only one of many phenomena which are sometimes associated with the occurrence of tropopause folds and occluded fronts. Chaotic changes and variances in adiabatic rates above and below these tropopause features are to be found anywhere around the planet and especially along the overlapping margins of separate tropopause layers. Adding even more complexity and chaotic adiabatic lapse rates is the poorly observed and transitory existence of secondary tropopause layers above the principal tropopause layers. Few of the idealized models even reveal the existence and complexities of the overlapping tropopause layers, much less the rarely researched and little understood secondary tropopause layers. In any event, substantial variability in adiabatic lapse rates above and below multiple tropopause layers is a planetary wide feature of the atmosphere and not just a transitory weather event associated only with local thunderstorm activity.
Sorry, but the tropopause is relatively thin and is typically not well defined. In fact, the WMO (World Meteorological Organization) has struggled over the years, mostly unsuccessfully, to formulate a lasting definition. Generally speaking, the tropopause is very loosely defined in part as a boundary layer of air above the troposphere and below the stratosphere which lacks certain chemical activities and in which the air temperature becomes virtually unchanging relative to the underlying troposphere and the overlying stratosphere. A tropopause layer is typically described as thin, because its thickness of only a few thousand feet and less is considerably thinner than the major atmospheric layers measured in tens of thousands of feet in thickness. Given the problems and expenses associated with extensive programs of upper air observation and analysis of tropopause layers, they remain poorly documented, poorly researched, and not well defined.
Re: #39
IIRC, I posted this to the blog last spring, but can’t find itpoorly implemented search engine.
Here’re two quotes WRT climate models from The Future of Everything: the Science of Prediction [originally entitled Apollos Arrow: The Science of Prediction and the Future of Everything] by David Orrell, mathematician, 2007:
“Einsteins theory of relativity was accepted not because a committee agreed that it was a very sensible model, but because its predictions, most of which were highly counterintuitive, could be experimentally verified. Modern GCMs [global climate models] have no such objective claim to validity, because they cannot predict the weather over any relevant time scale. Many of their parameters are invented and adjusted to approximate past climate patterns. Even if this is done using mathematical procedures, the process is no less subjective because the goals and assumptions are those of the model builders. Their projections into the futureespecially when combined with the output of economic modelsare therefore a kind of fiction. The problem with the models is not that they are subjective or objectivethere is nothing wrong with a good story, or an informed and honestly argued opinion. It is that they (GCMs) are couched in the language of mathematics and probabilities; subjectivity masquerading as objectivity. Like the Wizard of Oz, they are a bit of a sham.”
The track record of any kind of long-distance prediction is really bad, but everyones still really interested in it. Its sort of a way of picturing the future. But we cant make long-term predictions of the economy, and we cant make long-term predictions of the climate. Models will cheerfully boil away all the water in the oceans or cover the world in ice, even with pre-industrial levels of CO2 When models about the future climate are in agreement, it says more about the self-regulating group psychology of the modelling community than it does about global warming and the economy.
Philip–
See my earlier reply (49) which said:
Note that if you round 236.5267 Watts per square metre to two significant figures it’s the same as: 237 Watts per square metre. In fact, if you round to three significant figures, 236.5267 becomes 237, and so the two results are identical.
As you only started with 3 significant figures, you generally don’t report numbers to 7 significant figures.
So, yes, you have just demonstrated what I said. The differences are small, and generally speaking, at a level where you get the same answer after rounding.
Correction to a misleading typo:
#70, Willis:
I think I’ve seen your model before in a flat configuration, but it’s actually easier to understand in a spherical configuration as you’ve shown it.
Looking at a sequence of models:
0) No shell, ball of radius R_0, temperature T_0(0):
T_0(0) = a/sqrt(R_0), where a = (Power/(4*pi*SB))^0.25
1) 1 shell, ball of radius R_0, temp T_0(1); shell of radius R_1, temp T_1(1):
T_0(1) = a*2^(0.25)/sqrt(R_0)
T_1(1) = a/sqrt(R_1)
2) 2 shells, ball of radius R_0, temp T_0(2); shell of radius R_1, temp T_1(2); shell of radius R_2, temp T_2(2):
T_0(2) = a*3^(0.25)/sqrt(R_0)
T_1(2) = a*2^(0.25)/sqrt(R_1)
T_2(2) = a/sqrt(R_2)
And in general, for n shells, the temperatures are:
T_k(n) = a*(n+1-k)^(0.25)/sqrt(R_k)
The ratio of the temperature on the surface of the ball to that on the outermost shell is:
T_0(n)/T_n(n) = (n+1)^(0.25)*sqrt(R_n/R_0)
So this looks like a GHE, because the outside is cooler than the inside. Indeed, you can fit it if you want:
T_0(n)/T_0(0) = (n+1)^(0.25)
#86, continued:
The difficulty with this as an explanation of the GHE is twofold:
– Evidently, nothing in the derivation depends on radiation frequencies, so it cannot explain anything that does. I think we are reasonably confident that the IR band is important in the temperature increase. But if you want to take that as a “to be proven”, we go to the structural problem:
– The value n is a free parameter without any physical meaning in the real atmosphere. In fact, these metal shells don’t exist, and yet the precise number of them is extremely significant in the application of the model. To what should they correspond in reality? and with what explanation?
I think we are left with an elegant explanation of how some heat shields work.
Willis #81, lucia #84
So you classify my calulation as an example of accurate but wrong?
To see the complexity of the tropopause and read of the difficulty in formulating an official definition, see
Click to access KEY3-tropopause-legras.pdf
This might not be the most authoritative source, but here is an illustration which is more clear in the original reference.
The caption to the sectional diagram reads
Instantaneous height-latitude cross section of potential vorticity along a single longitude (55W), with the tropopause marked (in black) as the 2PVU contour. Courtesy of H. Wernli, ETH Zurich
I would like to thank Willis for actually teaching me something about thermo…er, That Which May Not be Spoken. His model (especially the diagram) gives a bit of insight into the counterintuitive-to-the-newbie aspects of the subject.
#88, Philip:
My calculation of #86 takes the spherical geometry into account explicitly.
T(0)_0 = -18 C (but you need to convert it to K)
Willis, Curt and Lucia,
thanks for your patience – I get it now! 🙂
Neal King, thank you for a clear mathematical exposition of the principles.
However, I must respectfully disagree when you say:
You are correct that this is a simplified model. However, that does not mean it can take any form. I propose that a feasible two “shell” model looks like this:
Figure 1: Depiction of the major energy flows in the global energy budget.
Note that for a multi-layer model to work, there has to be little thermal connection between adjacent layers. The gap then is bridged mostly by radiation. One layer is the lower stratosphere, and the other is the lower troposphere. As there is little thermal interchange across the tropopause, this fulfills the condition of layer isolation.
It also fits with the relative temperatures. The radiation from the stratospheric layer (147 w/m2) equates to a blackbody temperature of about minus fifty degrees … which (see graph in Steve Mc’s original post) is about the temperature of the lower stratosphere. And it puts the tropospheric average emission level at about freezing, which makes sense to me although I can’t exactly explain why.
Finally, as you point out, there is not any physical existence to the “shells” or “layers”. However, they are still very valuable abstractions. For example, there is no physical reality to something called the “centre of gravity” of some object, either. But we use this imaginary “centre of gravity” nevertheless, because mathematically it allows us to simplify a very complex situation. In the same way, we can analyze the energy budget using the mathematical abstraction of the two layers and the transfers between them. The fact that there is no point source or thin layer with an “average” radiation temperature in no way prevents us from using those abstractions to calculate average properties of the system.
Regarding an arbitrary number of layers “n”, I use the two-layer model because it fits and works, and it fulfills the physical requirement of thermal isolation between layers. It’s an Occam’s razor kind of decision. It is the simplest of possible models, and makes no bones about being anything else.
I have posted up an excel spreadsheet here that allows you to play with the two-layer model.
Best to all,
w.
Due to the discontinuous nature of the multiple tropopauses, isentropic flows, and their relationships with the formation and function of jet streams among other significant atmospheric structures; there is a substantial and variable flow of chemical species and thermal energy between the troposphere and the stratosphere associated with the discontinuities between tropopauses. Consequently, the assumptions of thermal isolation are invalid and the unreal models reliant upon such assumptions are fundamentally wrong and invalid with respect to reality.
#93, Willis:
I accept the model as a heuristic device, that shows that layering can result in an increased temperature at an “insulated” level. I can’t accept it as a physical explanation of what is really going on. (I guess D. Patterson feels somewhat similarly.)
btw: I notice that in the section that you have cut & pasted, the comparison scenario, the cells contain actual numbers instead of the functional expressions in the “live” section. How did you do that? I would find that very useful in other applications.
re 95 paste special option
Without leaving the planet, Mr R W Woods tested the back-radiation ideas proposed by Willis Eschenbach (70 ) about a century ago.
He built two identical greenhouses, one made of glass which absorbs most of the incident infra-red radiation, and one of rock-salt which absorbs very little.
Without the greenhouses, the surface would absorb and re-radiate W watts per square meter from the sun. Conventional wisdom at the time suggested that the glass greenhouse would absorb upward radiation from the interior, and radiate back half of what it received. The rocksalt greenhouse would allow the W watts to pass through.
Woods expected the glass greenhouse to warm exactly as Mr Eschenbach propsed for his steel enclose planet. At equilibrium, the incoming radiation per square meter would be W, the upward radiation from the interior 2W, and radiation from the glass W out and W back.
The temperature increase over and above the rock salt greenhouse interior would be proportional to the fourth power of the interior temperature (Stefan-Bolzmann), which is the fourth root of 2, or 18.9%. Sadly, it was not so: there was no detectable difference between the greenhouses.
You can still find this explanation for the greenhouse effect (planetary and terrestrial) on the internet.
Woods concluded :
Is it therefore necessary to pay attention to trapped radiation in deducing the temperature of a planet as affected by its atmosphere? The solar rays penetrate the atmosphere, warm the ground which in turn warms the atmosphere by contact and by convection currents. The heat received is thus stored up in the atmosphere,remaining there on account of the very low radiating power of a gas. It seems to me very doubtful if the atmosphere is warmed to any great extent by absorbing the radiation from the ground, even under the most favourable conditions.
I do not pretend to have gone very deeply into the matter, and publish this note merely to draw attention to the fact that trapped radiation appears to play but a very small part in the actual cases with which we are familiar.”
You can find the whole experiment desribed in the Gerlich and Tscheuschner paper, which regards all radiative explanations for the enhanced surface temperatures as nonsensical.
Tropopause: According to wiki, the WMO defines it in terms of lapse rate, beginning at “the lowest level at which the lapse rate decreases to 2 °C/km or less, provided that the average lapse rate between this level and all higher levels within 2 km does not exceed 2 °C/km.”
They say it can be “a dynamic definition of the tropopause is used with potential vorticity” at 1.5 or 2PVU surface.
Another is “It is also possible to define the tropopause in terms of chemical composition. For example, the lower stratosphere has much higher ozone concentrations than the upper troposphere, but much lower water vapor concentrations, so appropriate cutoffs can be used.”
But they don’t give the cutoffs. They do mention it’s not a hard boundry, and it of course varies in height (starting at about 5 miles at the poles and 11 miles at the equator).
I’d say based upon this, the tropopause is where the lapse rate is less than 2C/km at the bottom and greater than 2C/km at the top.
So let’s see if I get this straight. A steel band thin enough to abosorb bascially no energy would result in the surface having double the energy level due to the band reflecting back the original amount of energy, which it also sends that same amount of energy out the other side.
Seems counter-intuitive. It would seem it would send half each direction.
100space 100back
D. Patterson says:
Perhaps before you start rubbishing the model, you might actually take a look at it, yes? If you had, you would have seen that the flows across the tropopause are explicitly represented in the model.
Yes, there is a flow between the stratosphere and the troposphere. It is, however, much smaller than between adjacent parts of either the stratosphere or the troposphere. The fact that there are multiple tropopauses does not mean that there are large flows across them. Finally, I did not say there was no flow across the tropopause. The assumption of relative thermal isolation is valid, and cross-boundary flows are handled in the model.
w.
Bravo Willis.
Possibly related to the top of troposphere being the upper limit of cloud formation (liquid/solid phase change)?
Fred Staples, the experiment by Wood is discussed in
Effect of Infrared Transparency on the Heat Transfer Through Windows: A Clarification of the Greenhouse Effect
In other words, Wood’s observations were correct … but his conclusions were not. Or to quote Wood himself,
w.
Sam U, you say:
Sam, take another look at my first diagram above. It does send half in each direction, 235 w/m2 goes inwards, and 235 w/m2 goes outwards.
w.
So you’re saying with the crust releasing 235, since the energy source continues providing the energy, the reflected radiation of the steel shell warms the crust another 235. So from 235 we get 705.
Weird.
May I ask what temperature a hollow sphere (made out of a radioactive material) that radiates at 235 Wm/2 would be compared with a solid sphere?
Surely a hollow sphere the temperature will rise exponentially, according to this analysis. The inner side initially pushes out 235 Wm/2 and recieves 235 Wm2.
Wait a second. The steel shell doesn’t just reflect the radiation back, it would heat up. Wouldn’t it? How hot does a 1 cm thick steel shell get when it gets 235 w/m2? How hot does the outer crust get if it’s giving off 235 w/m2? How does the heat get transfered if there’s no atmosphere?
Something’s not adding up and I don’t know what.
Gerlich and Tscheuschner is only good for fishwrapper. Woods himself has already stated his experiment was worthless. Why they would include work that the author himself had written off in their paper is beyond me.
Good thread this. But my question is, has anyone in the scientific world got a big container with a black floor, transparent to IR radiation, and filled it with various air contents and CO2 concentrations, then let it sit in the sunshine or used a sun simulator (like for satelite testing) and produced a relationship for C02 v T with pressure, water vapour content etc? The walls have to be transparent to IR so the only change in temperature is due to gas composition.
It might be useful in greenhouse effect discussions. Modelling is one thing but an experiment is always better.
MC
The atmospheric greenhouse effect depends on a temperature gradient with altitude. Any small enclosed structure will be essentially isothermal. Also, the path lengths for significant energy absorption and emission are measure in 100’s of meters to kilometers. But we don’t need all that. We have measured IR emission and absorption spectra looking up, down and sideways from altitudes ranging from the surface to high orbit. The measured spectra agree very closely with spectra calculated from atmospheric radiative transfer theory. What happens to any excess energy absorbed by an increase in GHG concentration is the real question.
Some work on real greenhouses with partially IR transparent walls (polyethylene film) has been done and IR transparency apparently increases thermal efficiency by about 13%.
Your link, Mr Eschenbck (101) cost me 10 dollars, but it was worth it. The paper is only 3 pages long, but it repays careful study. Like Woods, Silverstein considers the cases of greenhouses transparent and opaque to infra-red radiation. Unlike Woods, (and in the best traditions of modern climate science), they do not appear to have actually built and measured the greenhouses.
However, their conclusion is that “ there certainly is an effect on the nature of the heat transfer associated with the IR opacity or transparency of a thermal insulating winow. However, this effect is more in the component mix of the different transport mechanisms than in the net thermal transport”.
The opaque glass is warmer than the transparent glass because it absorbs radiative heat from the interior. However, there is little effect on the overall heat transfer because the warmer glass loses convective heat more rapidly.
In other words, the temperature of the interior, which is where we live, is not affected by the greenhouse material, which is what Woods concluded.
Silverstein comments on Woods’ results:
“the sum of the convective and radiative losses is not changed appreciably by going froman IR-opaque cover (glass) to an IR transparent cover (rock salt or polyethylene)”
Nowhere in the text, or the equations, or the heat transfer factors, the Q’s, is there any back-heating factor Q rad (glass to room). In this they are absolutely correct, because any such transfer would contradict the second law of thermodynamics (the glass is colder than the room).
In their extrapolation to the atmosphere they are less modest than Woods. They view the atmosphere as a blanket, with (presumably) the greenhouse gasses analogous to the opaque glass. This effect must exist to some extent because of the vibrational modes of tri-atomic molecules. If this effect were significant, and not inhibited by saturation (another century old experiment) the increase in troposphere temperature would be greater than that of the surface as greenhouse concentrations increase. Sadly, (measurements again), it is not.
Another words, Willis Eschenbach, glass (and steel planetary enclosure) radiate heat according to their temperature, not according to adsorbed IR radiation. For glass, its temperature is close to temperature of outside air, for steel enclosure its temperature is close to “temperature” of outside vacuum. There is no back radiation when IR adsorbtion is involved. Only reflection produces back radiation.
The closest thing physically possible to your planetary experiment is enclosure of semi-reflector material. In that case half of emitted by planet IR will be reflected and indeed will warm the planet. In Earth atmosphere it is exactly what cloud cover is doing on cold night, preventing appearance of morning frost on the surface. Clear air does not prevent morning frost.
Such mechanics, based on interfence reflection, not simple adsorption, to substantially increase visible light output of halogen bulbs, was recently patented and used by GE. Simple IR adsorbtion/reemission does not cut it.
For details Google “HIR bulb” or “dichroic bulb”.
You can do the same math as Willis E. by postulating a layer with different absorption behavior for short wavelength incident solar radiation and and long wavelength emitted thermal IR, plus Kirchhoff’s Law (absorptivity = emissivity). For a single layer model in the limit of zero absorption of incident radiation and complete absorption of emitted radiation from the surface, the surface receives (and emits) twice the intensity of the incident solar radiation, exactly as in the case of the steel shell with an internal heat source.
The earth’s weather machine is an engine powered by light. High frequency radiation from the sun is absorbed by the base collector of the planetary surface and low frequency radiation is exhausted to space by the atmosphere’s external envelope. Because the planetary system collects and exhausts radiation, it is legitimate to consider the total fluxes involved using the irradiance metric of watts per square metre.
The particular atmospheric model supplied to planet earth comes fitted with a double phase change supercharger tuned for additional power output in cold environments. Vapour to liquid to solid water phase transformations. What is disingenuous about the heuristic model supplied by Willis is that the latent heat flux can only be achieved by movement of water carried aloft in ascending bodies of air, so this flux is dependent on the vertical translation of the water mass that forms clouds. The diagram #93 Fig 1 shows no return of mass to the datum.
The key feature of the cloud condensation and freezing processes, which drives the weather machine, is the physical separation of the components, water and air, by the precipitation mechanism. This mechanism dries the ascending air parcel and ensures that the lifted air can only return to the surface following radiative cooling to space. The water of course falls quickly to earth as rain, hail or snow. Atmospheric radiative cooling to space is a longer term process than that of a precipitation event, hence high pressure cells have a greater areal extent in our atmosphere than low pressure precipitation systems.
Like all engines, this planet’s weather machine achieves work through the movement of mass. In the case of the water this work supplies all the power that drives the planet’s total hydrological cycle including the glacial component. For the air, the movement of mass is the wind that interacts with the planet’s surface boundary and provides the dominant motive power of the ocean’s waves and currents. The motion of water and of air and the return of these masses aloft to the datum surface converts potential energy to kinetic energy, ultimately releasing heat within the atmosphere.
Suppose we consider the fluxes in the heuristic diagram #93 Fig 1 as vectors and define all down fluxes as positive vectors and all up fluxes as negative vectors. Does this help our thoughts? At the external boundary the incoming flux of HFR = +342, Albedo = -105 and the outgoing flux of LFW =-237, Vector sum equals zero. But does this concept apply for the internal components of the machine? Is there (should there be?) vector flux balance within an engine?
Suppose we modify the original floor lit planet and steel shell model #70 and call in the Las Vegas Hotel Decorators to fit a mirror ceiling to the underside of the enclosing metal shell. What effect does this have? We now observe an emitting lit floor and an image of the lit floor above us bring back light down from the ceiling, there is now more light in the room, a well known effect of a mirrored wall. So let’s now fit a mirrored floor too. How many sources of light do we now observe? 2? 4? 6? 8? an infinite series? What about the intensity of these additional sources? Are they all equal or does the inverse square law apply, to say nothing of transmission loss?
Why this may all be in good fun and have nothing to do with the concept of the green house gas effect, IMO it goes to the heart of the matter, heuristic diagrams cannot be used for engineering analysis.
DeWitt 108
The change in relative intensity of spectral lines can tell you the temperature of the molecule and this has been established over many years of atomic physics characterisation. But it doesn’t matter if the molecule is sitting in a vacuum tank or in the atmosphere, as long as you have a sufficient amount of molecules you can get a signal. WHat I am suggesting is by using the spectral intensity of CO2 (and direct temperature measurements) in a large but controlled environment (a thermal gradient can be applied to the tank in vacuum- this is how satelites are tested) and by varying the CO2 concentration, at least the logarithmic surface temperature forcing relationship i.e.using the bottom of the tank, can be established.
This can be extrapolated to atmospheric concentrations if this is needed. Yet it appears this information is not readily available or is quoted anywhere. You say, and I agree, that a smaller lab set-up would result in an isothermal temperature in the tank but surely this would change if CO2 concentration was changed and the bottom of the tank (a suitably absorbing material) would also change in temperature. Would this not act like Willis’ sphere? Adding more C02 to the tank would increase the insulation to the surface.
Willis, while your steel greenhouse is interesting and has got some people thinking and some others confused, it is not close enough to the earth’s atmosphere to enable us to visualize how it would apply to many of the atmosphere’s features. If we construct a planet with a perfect greenhouse of three layers one meter apart perhaps a few other influences can be examined.
The three layers of the greenhouse are one molecule thick, perfectly transparent to visible light, and absorb all IR radiation then immediately reradiate the energy in the infrared and are ten meters apart. If we illuminate the planet with 100 watts/sq. meter, the outer layer will radiate 100 watts/sq.meter to space when the system is in equilibrium. Since it also will be radiating 100 watts/sq. meter inwards, it must be receiving 200 watts/sq. meter. Since the outer layer receives no radiation from the exterior, the middle layer must be radiating 200 watts/sq. meter outwards. Working inward we find that the middle layer, inner layer and the planet are radiating 200 300 and 400 watts/sq meter outward respectively. This will not be surprising to any one who understood your steel greenhouse
The Earth receives infrared radiation as well as visible. If we add 100 watts/sq. meter infrared to our perfect greenhouse world, what will the result be. All the incoming IR radiation will be absorbed by the outer layer. At equilibrium the outer layer thus will radiate 200 watts/sq. meter out to space and also inward. Since it now is receiving 100 watts/sq. meter from the outside it is receiving 300 watts/ sq. meter from the middle layer. The middle layer is emitting a total of 600 watts/sq. meter. Since the outer layer is emitting 200 watts/sq. meter inwards, the middle layer is receiving 400 watts/sq. meter from the inner layer. Proceeding inward with the same procedure we find that the inner layer and the planet are radiating 400 and 500 watts/sq. meter respectively.
If we add a hundred watts/sq. meter UV to the incoming radiation and let the middle layer absorb the UV while the outer layer transmits UV, we find that the planet emits 700 watts/ sq. meter and the inner to outer layers are emitting 600, 500, and 300 watts/sq. meter outwards. One hundred watts/sq meter has raised the amount the plant radiates by 100, 200, or 400 watts/sq. meter, depending on where it was absorbed.
We now can transfer our observations to the earth’s atmosphere. The effect of incoming radiation on the earth depends on where it is absorbed. All warms the earth, but the radiation that reaches the earths surface will warm it more. Also one watt/sq. meter increase in the radiation reaching the earth’s surface is going to increase the radiation from the surface by more than one watt/sq. meter.
Aerosols have an influence on radiation that depends on their altitude. Dust from a volcano that absorbs 1% of the radiation that would otherwise reach the surface, will have a greater cooling effect than the same dust absorbing 1% of the radiation near the surface, where most of the greenhouse effect is above it. Dust of course also will absorb outgoing infrared radiation, but to see just how that effects the earth, we will have to introduce a few imperfections in the perfect greenhouse, which will be the subject of another post.
The radiation will heat metal. The metal will re-radiate the heat out equally, the only thing to determine is how much does the planet’s internal radiation out from the crust cause the atmosphereless space to transfer to the metal in the form of heat? That tells us what the steel provides as heat out to space and back towards the planet.
What is the transfer mechanism in the space that was recently vaccuum? How much heating is taking place; some of the energy has to be used to heat the metal. If 100C comes out of the planet, what does it heat (say) 1 CM of steel 10K high around the planet?
To Steve
You say you have been searching for a good explanation of the enhanced greenhouse effect, and have been unable to find one in Houghton or the IPCC publications. I don’t think you ever will, because I believe there is no such effect, with the possible exception of the so-called “wings effect” which might operate at low altitudes, but only in the absence of water vapour.
The explanation attempted by the Real Climate site seems to rely on events at very high altitudes, but I have studied this very carefully, and I think it is simply wrong. It works in the opposite way. Added CO2, indeed, would cause the emitting layer to move upwards where it is colder, but this would reduce the extra emission to space, and so cancel out the effect.
More later if there is any interest.
AE
You have some interesting ideas.
I’d like to know what you mean by ‘enhanced GHE’, so we can start on the same page. I take it you mean the effect due to the extra CO2 over and above the 280 or 300 ppm?
However, I disagree with your first paragraph. I agree that the wings do play the main role, but believe that they do so even in the presence of water-vapor.
You mention you have looked at the high-altitude issue very carefully. I would like to exchange ideas on this topic. My own study suggests that cooling at the top of the troposphere should result from the CO2 increase.
Re #117 Pat Keating
Thank you for your prompt reply.
Yes, we mean the same thing by the term “enhanced GH effect”.
OK, let’s leave the “wings” for now, and return to it later. It is certainly worth further consideration.
My main concern at present is with the Real Climate “Saturated Gassy Argument” (SGA) dealing with high altitude effects. I think their conclusions are completely the opposite of what is really happening. I have repeatedly tried to show them my concerns about this, but they have declined to accept further details from me. It appears that they simply don’t want to know, presumably because it goes against their strongly held “beliefs”.
I am encouraged by your last paragraph. I, too, believe that it is the function of CO2 at high altitudes to provide the means (photons) for the Earth to emit its energy to space in order to maintain energy balance, that is a cooling effect.
Please refer also to my earlier posts in this thread at #62 and #69. I’m afraid I posted both of these at the wrong time, in the middle of another discussion, and both seem to have been overlooked. One little problem with #69, though. A minus sign should have appeared several times in the latter part of the post, but it appeared as a square. The term should have read as (1 minus s).
One point I have in agreement with the SGA is that extra CO2 will cause the emitting layer to rise to higher, colder altitudes. But that’s as far as I agree with SGA. So, rather than an actual cooling, with the emitting layer being at colder temperatures because of the higher altitudes, the emission rate will be reduced to re-establish energy balance.
So added CO2 does not cause any enhanced GH effect.
Incidentally, this suggests that the emitting layer will rise and fall with extra and less CO2 respectively.
I have also had a quick look at the brightness temperature of the CO2 band, and it seems quite easy to reconcile the observed 215K with the overall amount of energy available in the atmosphere, according to the work of Kiehl and Trenberth, http://www.cgd.ucar.edu/cas/abstracts/files/kevin1997_1.html
I’m not familiar with the ‘saturated gassy’ argument (I don’t usually get much out of visits to RC), but I’ll put that aside for the moment.
I have to leave for a while, but I will look at the posts you reference and get back to you.
118 AEB
I have started looking at your #62 post, and am still doing so. However, you lost me here:
This doesn’t make sense to me. Can you elaborate, hopefully accepting that the mixing ratio for CO2 is constant from the surface to well into the stratosphere.
There are all kinds of saturated gassy arguments on RC.
Re #120 Pat Keating
I think we agree that the Earth is kept in energy balance by the cooling effect of photons being emitted from the high altitude regions of the atmosphere. So for a given, fixed energy input from the Sun, the output is also fixed. Therefore, the number of CO2 molecules emitting photons which escape to space is also fixed. Again, therefore, it matters not if the CO2 concentration is increased. All that happens is that the PROPORTION of the total CO2 participating in the process is reduced in order to keep the participating NUMBER constant. This is achieved by the emitting layer moving to higher, colder altitudes.
As far as photons emitted DOWNWARDS are concerned, they can be totally discounted. The excited CO2 molecules concerned got their extra ehergy of excitation from the Earth/atmosphere system in the first place, and then it is returning there by collisional decay. So nothing has changed within that system.
AEB
There is another way, the emitting layer doesn’t have to move upwards — and there isn’t anything to drive such a response.
If you suddenly add some more CO2 molecules there at the local temperature, there will be a temporary increase in IR emission. This will cool the emitting layer until its output matches the balancing level. It cools in place.
I suggest that this is how the emitting layer responds, because (a) the natural response is to emit more and cool down, not to rise, (b) the satellite data shows this cooling at the same, say, 14km level.
Re #123 Pat Keating
Good point. Thanks for that. You could well be right. Please could you give me a reference to the satellite data?
So I think we have here the basis for a very simple model of the natural greenhouse effect, and which precludes any enhanced effect, except for the “wings” which still need considering.
But you try convincing anyone at Real Climate about that. They are incredibly attached to the “Saturated Gassy Argument” about high altitudes, and seem to think that this approach is more credible than the “wings” approach. Don’t bother to try making an argument they don’t like, because you won’t get very far.
More about the wings later, if I get the chance.
AE and Pat,
I see what y’all are getting at, but. This is my GHG analogy. What the heck y’all deserve a chuckle.
Imagine driving into a heavy rain in your car. You see the storm but can’t see trough it. As you enter the storm, you can’t see squat in front of you so you slow down. In the rear view you can see pretty good. In the middle of the storm, you can’t see in front or behind you, you slow way down. As you past the center you can see better up front and speed up a little. Can’t see a thing but rain in the rear view. As you past the storm you resume normal speed and see all the cars slowing down in the rear view as they enter the storm.
The middle of the storm is an area of equilibrium. It is just as much a pain to drive in either direction. Before the middle it is easier to back up, etc.
With increase CO2 and H2O that equilibrium point would move down not up. Above the equilibrium point it is easier to get out not in. Below, the opposite.
The interesting thing is looking at the tropical Troposphere. When surface temperatures increase, the Trop temp increases very fast as predicted. When surface temperature reduces or at least stop rising, the trop temp decreases much faster than predicted. Why?
125, Capt’n:
As predicted by what?
Jae, by the GCMs, the tropical troposphere should warm faster than the surface temperature by anywhere from 1.4 to 2.0 times depending on the model. This is John Christy’s big problem with the models to the best of my knowledge.
Let me add to this that when Eli Rabett was trashing our host after the Hansen ABC predictions post, he mentioned that the TLT increased as expected during El Nino events. Eli did not mention the cooling of the TLT following El Nino events. Hmm?
124 AEB
The satellite data link is to one of John Christy’s papers, Douglass et al, discussed on one of the other threads:
Click to access climatemodel.pdf
See figure 1. (I wasn’t able to find it in the journal, so maybe it hasn’t actually come out yet).
As far as the wings go, I’ve actually done an analysis, based on high-resolution transmission spectra, but I’d certainly like to hear your views.
125 capt
I’ve heard that analogy before on CA, maybe in one of your posts(?). What happens if you get mud on the rear window?
Re #129 PK
I shall have to go through my “wings” work again and try to firm it up a bit. This might take a few days. Please would you consider posting your analysis in the meantime?
My analysis of the high-resolution spectra is detailed, and somewhat long. I’m pretty sure that Steve M would be unhappy if I posted it.
Re #132 PK
OK, but what are your conclusions?
Pat K.
You need to wash your car more often or global cooling has started.
133 AEB
Two of the conclusions are:
1. CO2 contribution to warming at 300ppmv is about 23% of the total
2. Climate sensitivity is about 1.2C (mostly from a widening of the 15u continuum), or about 16% of the contribution from the first 300ppmv.
133 AEB
I forgot to say in 135 that the climate sensitivity quoted is the ‘bare’ sensitivity, without positive or negative feedbacks.
The steel shell problem: First of all, if the planet is radiating 237 W/m2 it must have a very energetic energy source inside or it will cool down very fast. The geothermal heat from the earth is many orders of magnitude smaller. It would have to have something like a layer of very active radioactive minerals just under the surface. Any insulating material placed on the surface would greatly increase the temperature of the surface in order to keep the heat flowing outward. So this is not a good analogy to the real earth. Placing a steel shell above the surface will, neglecting 2nd order effects from changes in surface area, increase the temperature of the surface enough to double the radiation because the steel shell radiates equally in and out and enough additional radiation from the surface is required to balance energy. Assuming an initial surface temperature of 300K the surface temperature would increase from about 300K to 357K following the 4th power law of radiation. The steel shell would assume the 300K temperature. If we lived in a vacuum and used radiation emitting heaters to keep warm we would live in rooms constructed with multiple shells of material as insulation.
That Houghten(1995) point is interesting. I had thought that the CO2 “greenhouse” effect was mainly the loss in the “wings” of the infra-red spectrum from surface radiation, causing surface heating, evaporation, wild weather etc etc. However the quote implies that the major contribution comes from the extra CO2 in the upper atmosphere “blanketing” radiation from the mid-upper troposphere, causing an increase in height of the troposphere, causing an increase in surface temperatures because the more-or-less fixed convectional lapse rate implies an increased surface temperature. So I wonder which of these two effects is of most concern today.
My understanding of the science is that a layer of the atmosphere cannot actually radiate unless it contains some “greenhouse” gases. Thermodynamically, emissivity must be equal to absorption. So the stratosphere is actually cooler with additional CO2, largely because ozone absorbs solar UV radiation, and transmits this energy by conduction to CO2, which emits LW radiation, while N2 and O2 are largely inert. The atmosphere is too thin to absorb much of the LW radiation from the Earth below. So more CO2 in the stratosphere means more LW emission, so it has cooled by 1-2 degC.
The upper troposphere receives energy in the form of convection, LW absorption, and perhaps ozone UV absorption, and transmits this power through LW radiation, from clouds, water vapor, and CO2. Extra CO2 will help this transmission, as well as absorbing more LW radiation from below. I believe the temperature and vertical position of the upper troposphere has not changed? If so, then the physical effects of CO2 in the mid-upper troposphere balance, and the “radiation blanket” mechanism must be questioned.
3 Trackbacks
[…] This could be further quantified in future posts. Contrasting the heat engine model with the ‘steel shell‘ model of Willis Eschenbach, and another model of greenhouse warming applied to ice beads […]
[…] temperatures based on radiative equilibrium in the atmosphere: Willis Essenbach’s ‘steel greenhouse‘, Miskolczi’s semi-transparent atmosphere model, and finally the ‘received’ […]
[…] at top of atmosphere The following are different three constraints: 2.1 0 = Ed – Eu — the steel greenhouse, top of atmosphere constraint. 2.2 0 = Su – Ed — the Kirchhoff’s law, IR radiative […]